BRAINS IN BRIEFS
Scroll down to see new briefs about recent scientific publications by neuroscience graduate students at the University of Pennsylvania. Or search for your interests by key terms below (i.e. sleep, Alzheimer’s, autism).
How Brain Injuries Affect Sleep-Related Neurons Differently in Male and Female Mice
or technically,
Mild Traumatic Brain Injury Affects Orexin/Hypocretin Physiology Differently in Male and Female Mice
[See original abstract on Pubmed]
Rebecca Somach was the lead author on this study in the Cohen lab. Rebecca is currently doing her postdoctoral fellowship at Swarthmore College. Her current research uses planarians as a model system to understand how pesticides affect developmental neurotoxicity. She's interested in helping students understand neurobiology and helping them achieve their research goals.
or technically,
Mild Traumatic Brain Injury Affects Orexin/Hypocretin Physiology Differently in Male and Female Mice
[See Original Abstract on Pubmed]
Authors of the study: Rebecca T Somach, Ian D Jean, Anthony M Farrugia, Akiva S Cohen
A traumatic brain injury (TBI) occurs when an external source, such as a strong hit to the head, damages the brain. TBIs can be mild or severe, with mild TBIs (mTBI) being especially common and accounting for a large portion of TBI cases. Both TBI and mTBI can negatively impact brain physiology and lead to problems like sleep issues. Surprisingly, despite a large portion of TBI patients experiencing disordered sleep, there is very little research focusing on how these injuries may affect neural circuits that regulate sleep. Understanding the link between TBI and disrupted sleep is an important step in improving treatments for TBI patients.
Orexin/hypocretin neurons are specialized brain cells found in a brain region called the Lateral Hypothalamus. These neurons play a critical role in sleep and wake regulation. When they are not working properly or if they die off, it can lead to sleep-related issues, such as narcolepsy (a disorder where people fall asleep unexpectedly) or excessive sleepiness during the day. Some research suggests that TBIs can disrupt orexin, which could explain why many TBI patients have sleep troubles.
However, the Cohen lab found that sleep problems after mTBI can happen even when the number of orexin neurons does not decrease due to the injury. This raised an interesting and important question: is it the activity of these neurons after injury, rather than how many there are overall, that is important? To tackle this question, the Cohen lab looked at how active orexin neurons were after mTBI by labeling them for cFOS, a protein that is produced when neurons are active. They found that instead of orexin neurons dying off, the activity of the overall population was actually reduced (less cFOS in the population), suggesting that the number of neurons alone is not the only factor to consider after TBI. These findings were important, as only two other studies have looked at how orexin neuron activity changes after brain injury.
Rebecca Somach, an NGG graduate from the Cohen lab, decided to further expand on this work by incorporating other fascinating neuroscience techniques. These included electrophysiological recordings, which measure electrical activity in the brain, and a mouse that has orexin neurons tagged with a glowing protein, making it easier to study and see these neurons in the brain. Rebecca also wanted to examine the effects of brain injury might be different in male and female mice, since sleep can vary between sexes. This is particularly important because of the little research on the effects of TBI, specifically mTBI, on orexin in female animals.
Rebecca specifically examined whether mTBI changes how orexin neurons might respond to electrical signals or how often they fire in male and female mice. To do this, they used current clamp recording, a neuroscience technique used to see how neurons react when they are injected with electrical current. In female mice, they found that orexin neurons became more negatively charged after mTBI, which is a process called hyperpolarization. In contrast, they found that in male mice, orexin neurons had a reduction in action potential threshold after mTBI, making it easier for them to fire. These seemingly small changes in how neurons behave after mTBI can have a big impact on how they communicate to other areas of the brain.
While orexin neurons are found in the Lateral Hypothalamus and talk to neurons adjacent to them, they are also connected to many different parts of the brain. Because of this, Rebecca wanted to examine whether mTBI could affect the connections these neurons receive from other brain regions. Orexin neurons receive both excitatory and inhibitory signals. To only focus on excitatory signals, Rebecca recorded neurons while applying a chemical called bicuculline, that blocks inhibitory signals. This allowed them to study how only excitatory signals may have changed after brain injury in male and female mice. They looked at two types of excitatory post-synaptic currents - spontaneous (sEPSC) and miniature (mEPSC), which can be thought of as the orexin neurons’ responses to excitatory inputs from other neurons. In short, the stronger the EPSCs, the more likely the neurons are to become active. After injury, they saw that these signals were less frequent and weaker in both male and female mice, meaning that the orexin neurons were getting less excitatory activity.
Next, Rebecca did the reverse experiment and isolated only the inhibitory signals to examine if there were mTBI-induced changes. She did this by recording orexin neurons with AP5 and CNQX, which are chemicals that remove excitatory currents. Rather than looking at sEPSCs and mESPSCs, she then focused on recording spontaneous and miniature inhibitory currents (sIPSC, mIPSC) in male and female mice. After injury, she saw reduced time between sIPSCs, meaning they occurred more often. Interestingly, she only saw stronger sIPSCs and mIPSCs in female mice, which could help explain why sleep disturbances after TBI can vary depending on sex.
Overall, while previous research has shown an effect of mTBIs on orexin neurons, not many studies looked at how brain injury impacts the activity of these neurons. Rebecca addressed this gap by exploring how the activity and connections of orexin neurons are altered after brain injury in both males and females. She shows that mTBI significantly alters how orexin neurons respond to inputs from other neurons and that although these changes appear similar between male and female mice, the underlying mechanisms are not the same. Ultimately, her work provides insight into how brain injury may disrupt sleep-related circuits and informs us why TBI patients may experience disordered sleep.
About the brief writer: Diana Pham
Diana is a Neuroscience Ph.D. student in Dr. Brett Foster’s lab. She is broadly interested in how memories are encoded and consolidated in the brain.
Want to learn more about the effects of brain injury on sleep-related neurons? You can find Rebecca’s full paper here!
How does the brain transform our memories during sleep?
Or technically,
Memory reactivation during sleep does not act holistically on object memory
About the author: Liz Siefert
Liz is a 4th year PhD candidate working with Dr. Anna Schapiro and Dr. Brett Foster. She is interested in how our memories change and shift overtime, and the role of arousal states (wake, sleep) in these changes.
or technically,
Memory reactivation during sleep does not act holistically on object memory
[See Original Abstract on Pubmed]
Authors of the study: Elizabeth M. Siefert, Sindhuja Uppuluri, Jianing Mu, Marlie C. Tandoc, James W. Antony, Anna C. Schapiro
Whether you’re a human, dog, or fruit fly, we all sleep. There’s no doubt that sleep is essential, but why we sleep is still hotly debated. Many ideas have centered around the observation that sleep is important for strengthening key memories and putting them into storage. Neuroscientists believe this happens during sleep when the brain replays memories to strengthen them, a process called memory reactivation. Supporting this idea, numerous studies have shown that people tend to do better if they take even a short nap between learning something and being tested on it.
NGG student Elizabeth Siefert was fascinated by the process of memory reactivation during sleep, but wanted to better understand what impact it has on our memories. She noticed that sleep can’t just be improving memory overall, because that’s not how memory works. “As we go about our lives, our memories don’t always continue to improve,” says Siefert. “Often, they get worse, or they change in different ways. So really, across time, memory isn’t just improving, but it’s transforming.” Following on these observations, Siefert designed an experiment to understand whether sleep simply improves memory or rather has the power to transform it by strengthening some aspects of a memory while allowing others to fade.
Studying the sleeping brain
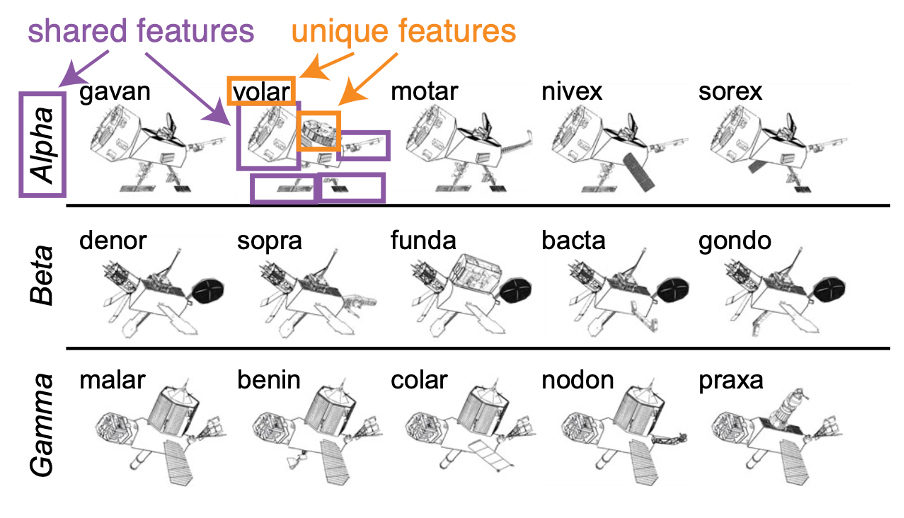
To probe the nature of memory transformations during sleep, Siefert and her team needed a memory test that allowed them to assess different aspects of memory. “Memories have lots of different features, so we wanted to know if memory reactivation has the power to act on different features of our memories in different ways,” says Siefert. She did this by asking participants to learn the identities of several satellites belonging to three groups. The satellites were created by mixing different parts so that the team could control the different features of a memory (Figure 1). Some shared features appeared on multiple satellites within a group, but never on satellites in other groups. Other unique features were specific to just one satellite, allowing the participants to identify it by name. This allowed the team to look at how representations of the individual versus the shared features were transformed in memory during sleep. Siefert asked the participants to learn the satellite names and groups, had them take a nap, and then tested how well they remembered the unique versus shared satellite features.
Just because participants took a nap doesn’t mean they were necessarily going to replay their memories of the satellites. That’s why Siefert stepped in to nudge their sleeping brains to replay the memories she was interested in. She did this using a method called targeted memory reactivation (TMR). During TMR, experimenters measure a participant’s brain activity while they sleep and look for moments when the sleeping brain is most likely to replay a memory. When those moments are identified, the experimenter plays a cue to remind them of a recent memory and encourage the brain to replay that specific memory. The cues are played softly enough that they don’t wake up the participant, and participants don’t know that the cues are being played, so any impacts of TMR are unconscious. “The best method we know in humans for studying memory reactivation in sleep is TMR,” says Siefert. “Our sleeping brain is already prioritizing certain information, and what we’re doing with TMR is biasing the brain to prioritize what we want it to.” In Siefert’s experiment, she played the name of some of the satellites the participant had just learned to encourage reactivation of those memories.
Figure 1. Satellites used in the memory task. Satellites belonged to three groups: alpha, beta, or gamma. The purple and orange boxes highlight the shared and unique features of the satellite volar. The purple boxes show volar’s features that are shared with other satellites in the alpha category, while the orange boxes show the features that are unique to volar.
Clarifying the relationship between sleep and memory
With all the pieces in place, Siefert was ready to ask how memory replay during sleep impacted different features of memory. Before the nap, participants tended to do a better job learning the satellites’ unique features compared to their shared features. In other words, they were better able to identify that a particular feature belonged to an individual satellite than that a particular feature was shared by satellites of a certain group. After a nap with TMR, that divide only widened. Siefert found that TMR increased participants’ memory for unique features while decreasing their memory for shared features. For satellites whose names were not used as cues during sleep, there was no impact on memory. This demonstrates that the effects were likely due to the reactivation encouraged by TMR. “That showed us that memory reactivation during sleep, it’s not just wholistically improving our memory, it has the power to act on specific features of our memory in very specific ways,” says Siefert. “It [can] even impact different features of our memory in different ways, such as improving some even at the cost of others.”
Why were certain features strengthened while others got weaker with reactivation? One possibility is that the cues used to trigger replay during sleep were the satellite names. This may have encouraged the brain to prioritize information about the individuals’ identity rather than their group membership. Future studies could use the group names as a cue instead and see if that nudges the brain to prioritize shared over unique features. Another possibility is the fact that people already tended to learn the unique features better before the nap. That prioritization may have carried over into their sleeping brains. “Your learning strategy and goals before sleep might bias what type of information the sleeping brain wants to reactivate,” says Siefert. “It’s possible that it was those learning strategies that led that information to be benefitted more by sleep.” Importantly, no matter what the explanation, it’s not the case that unique features will always be remembered over shared features following a night of sleep. Instead, a complex combination of learning goals and strategies likely shape exactly how memories are transformed during sleep.
Unbeknownst to the participants, Siefert was also testing how the way she presented the cues during sleep impacted memory. Sometimes she played the same satellite name over and over in a block, and other times she played satellite names intermixed with each other. Importantly, she always played the satellite names the same number of times during sleep, only changing in what order she played them. While both methods led to improved memory for unique over shared features, repeating the same satellite name many times in a row was more effective in transforming memory than playing the names in a random order. Siefert suggests that this may be evidence that reactivating memories in blocks helped the brain differentiate things in memory more than random reactivations.
The lab is already extending their results to understand more about the relationship between sleep and memory. Specifically, they’re interested in how sleep may help us take new information and incorporate it into older memories. “A study that we’ve run in the lab since [mine] is trying to understand what it looks like to learn new information that is aligned with things that you know from the past and how that new information can become integrated into older memories without totally overwhelming those old memories,” says Siefert. It seems that we’re only scratching the surface of what TMR can teach us about the sleeping brain’s relationship to memory.
What does this mean for everyday life?
After learning about these results, you might wonder whether students should give up on wakeful studying and just play their textbooks while they sleep. Unfortunately, it’s still not that easy. “Because [memory reactivation] doesn’t just improve memory and has this transformation component, if you took this device home and used it to play a textbook it’s not clear to me whether it would improve or hurt your memory of that information,” says Siefert. “You might need specific learning goals, you would need to think carefully about when you are delivering the cue, and you need to consider lots of other things.” It may be possible to design a system that could help a student study in their sleep, but we still need to learn a lot more about how TMR works before that will be possible.
Despite its complicated nature, some scientists are optimistic that they may be able to bring TMR to your bedroom. Rather than recording brain activity to target specific moments for reactivation, they aim to use audio recordings of movement during sleep to target the longer periods of sleep when they think replay naturally occurs. “For me in the lab it’s really important to target specific moments so I really know what my cues are doing, but in the real world that might be less important,” says Siefert. TMR is already being used in some clinical settings to help stroke patients relearn how to move their bodies, and Siefert suggested that it could one day be helpful for things like language learning if you’ve already learned some of the basics. For those who may be worried about TMR being used for brain washing or unconscious influence, Siefert says we aren’t capable of that now and may never be. “Your brain is already doing things and we’re just biasing it to little quick moments,” says Siefert. “We aren’t at the point where we can totally change the way that you’re thinking about something.”
Even in the absence of a TMR system on your nightstand, one takeaway is clear: sleep is important. “We don’t have a good understanding of why we’re remembering and forgetting certain things but knowing that the brain is selecting information means that that selection is probably important, and that selection is clearly happening during sleep,” says Siefert. “That means you should be getting good sleep, because you want to allow your brain time to process the information.” So next time you’re faced with the decision to stay up a little later studying for a test or prepping for a meeting, remember that choosing to sleep may be even more important than putting in that extra half hour of work.
About the brief writer: Catrina Hacker
Catrina Hacker is a PhD candidate working in Dr. Nicole Rust’s Lab. She is broadly interested in the neural correlates of cognitive processes and is currently studying how we remember what we see.
Learn more about the team’s memory reactivation study in the original paper.
Neurons in the brainstem promote REM sleep and trigger brainwaves that might cause dreaming
or technically,
A medullary hub for controlling REM sleep and pontine waves
[See original abstract on Pubmed]
Dr. Amanda (Mandy) Schott was the lead author on this study. As a researcher, Mandy is most interested in defining neural circuits, and how specific populations of cells communicate to generate essential human behaviors such as sleep.
or technically,
A medullary hub for controlling REM sleep and pontine waves
[See Original Abstract on Pubmed]
Authors of the study: Amanda Schott, Justin Baik, Shinjae Chung & Franz Weber
Rapid eye movement (REM) sleep is the sleep state that most people associate with dreaming, however REM sleep has many other essential functions. While REM makes up only about 20-25% of our nightly sleep, it is vitally important for memory, emotional processing, and other functions we have yet to understand. This is true not just for humans, but all mammals and maybe even birds and reptiles! To facilitate all these functions of REM, the brain is highly active during this sleep state. In fact, during REM sleep, brain signals look more similar to wake than non-REM sleep. Because of this, REM sleep is sometimes called paradoxical sleep because paradoxically, the brain is so active during rest.
Surprisingly, we still know very little about how the brain switches from low-activity non-REM sleep to high-activity REM sleep. Moreover, during REM sleep there are sometimes sporadic brain waves that seem to be important for normal brain function but whose precise role is still not totally clear. P-waves are one such waveform that is caused by lots of synchronous neuronal activity in the back of the brain, in a brainstem region called the pons. From the pons, P-waves travel forwards in the brain to brain regions important for forming and storing memories, and also areas involved in visual processing. These P-waves are interesting because they occur only during REM sleep, and are proposed to be involved in dreaming and the memory functions of REM sleep. A paper by recent NGG graduate Dr. Amanda Schott investigated two major unknowns in REM sleep research: 1) What neurons and brain regions are involved in generating REM sleep, and 2) What neurons and brain regions are involved in generating P-waves. Is it possible that one set of neurons could do both?
While we know of several brain regions in the brainstem that regulate REM sleep, most of them consist of inhibitory neurons, meaning they “turn off’ other brain regions to promote REM sleep. Dr. Schott, however, found a highly unusual group of excitatory neurons in part of the brainstem called the dorsal medial medulla (dmM). These excitatory neurons can “turn-on” other neurons they make connections with. These dmM excitatory neurons were only active during REM sleep, suggesting they may be involved in promoting REMs sleep. In addition, dmM neurons project their axons and send signals to the part of the pons that is known to generate p-waves. In fact, the dmM neurons were active at the same time the p-waves occurred suggesting that the dmM excitatory neurons could be involved in the generation of p-waves too! Dr. Schott next wanted to directly manipulate the activity of these neurons to see if they could cause transitions to REM sleep or cause generate p-waves.
Using a modern neuroscience technique called optogenetics, Dr. Schott was able to cause the neurons in the dmM to fire when a laser light was shined over them through an optic fiber. She simultaneously determined if the mouse was awake, asleep, or in REM sleep by measuring the mouse’s brain waves using electroencephalography, or EEG. She found that stimulating these neurons caused the mouse to enter REM sleep, and also increased the length of REM sleep episodes. Shining the laser light also caused a p-wave to be generated when the light was shined about 60-100% of the time when the mouse was sleeping. Experimentally reducing the activity of the dmM neurons also decreased the amount of REM sleep, as well as the amount of p-waves. Dr. Schott interpreted these findings as evidence that dmM excitatory neurons are critical for normal amounts of REM sleep to occur, and for triggering p-waves.
Overall, Dr. Shott’s work adds an important piece to the puzzle to our understanding of which brain regions can promote REM sleep. Her findings are an important first step in understanding which neurons generate p-waves which is ultimately necessary to understand p-wave function. This work will provide a foundation on which others (including the author of this piece!) can study the role of p-waves in REM sleep, and move closer to finally understanding how and why we dream.
About the brief writer: Emily Pickup
Emily is a 4th year PhD candidate in Dr. Franz Weber’s lab. She is interested in the biological functions of sleep. Specifically, she is interested in understanding the function of REM-specific p-waves. The large pontine waveform implicated in memory consolidation discussed in the brief above.
Interested in learning more about REM sleep and p-waves? See the original paper here.
A mouse model for autism and ADHD can mimic sex differences in sleep
or technically,
Hyperactivity and male-specific sleep deficits in the 16p11.2 deletion mouse model of autism.
[See Original Abstract on Pubmed]
or technically,
Hyperactivity and male-specific sleep deficits in the 16p11.2 deletion mouse model of autism.
[See Original Abstract on Pubmed]
Authors of the study: Angelakos CC, Watson AJ, O'Brien WT, Krainock KS, Nickl-Jockschat T, Abel T.
Patients with disorders like ASD/ADHD often have changes in the number of copies they have for a geneA unit of DNA that encodes a protein and tells a cell how to function. Typically, for each geneA unit of DNA that encodes a protein and tells a cell how to function there are two copies - one from each parent. Therefore, individuals with ASD/ADHD can have more copies, or fewer copies (also known as a deletion). One of these changes is a deletion in chromosomal region 16p11.2. People that have a deletion in this region are more likely to have ASD and ADHD. Previous research has shown that mice with a deletion in the 16p11.2 region show symptoms similar to ASD/ADHD like differences in brainThe brain is an organ that serves as the center of the nervous system in all vertebrate and most invertebrate animals. structure, cognitive ability, and communication. However, sleep problems remained largely unexplored, a problem that Christopher wanted to address.
Christopher observed that these animals were hyperactive, a behavior that is observed in individuals diagnosed with ADHD. He tracked all of the movements of the mice in their cages, observing an increase in activity in the 16p11.2 deletion mice throughout the day, and a robust increase during the dark (active) phase of their cycle. This led him to think that something may be altered in their circadian rhythms. To investigate this he monitored them for 24hrs and measured their sleep and activity to determine if it was normal.
He also examined their sleep cycles using polysomnography, which tracks brainThe brain is an organ that serves as the center of the nervous system in all vertebrate and most invertebrate animals. waves, eye movements, and limb movement during sleep. He wanted to know whether this was a problem of initiating sleep or maintaining sleep. He found that once the animals were asleep, they usually remained asleep for the same amount of time indicating that there was not a problem of staying asleep. On the other hand, once an animal was awake, it was usually awake for a longer period of time, indicating that it may have had trouble with initiating sleep. When Christopher further analyzed the data, he saw male mice with the 16p11.2 deletion spent a longer amount of time awake than regular mice. Coupled with his finding that these mice stay asleep as long as the regular mice, this suggests that they had a hard time falling asleep, rather than that they were waking up multiple times and having brief amounts of wakefulness. Interestingly, these disorders are more commonly found in males rather than females. Males are four times more likely to be diagnosed with ASD and three times more likely to be diagnosed with ADHD.
Issues with sleep in people that are diagnosed with autism or ADHD is a problem that needs to be addressed. Christopher asked whether we can use a mouse to model sleep problems in autism? He showed in his paper that the 16p11.2 deletion mouse can model sleep disturbances that are seen in humans. He is excited to see future work using this mouse model to uncover specific brainThe brain is an organ that serves as the center of the nervous system in all vertebrate and most invertebrate animals. circuits that may be involved and better treatment for sleep problems. Now that we have this experimental mode, we can determine if improving sleep quality will improve other psychiatric symptoms.
About the brief writer: Felicia Davatolhagh
Felicia is a PhD Candidate in Marc Fuccillo’s lab. She is a seventh year studying the impact of neuropsychiatric disease on synaptic connectivity and synaptic function.
Want to learn more about sex differences in neurodevelopmental disorders? You can read Christopher’s whole paper here.
How support cells in the brain support sleep.
or technically,
Endocytosis at the Drosophila blood-brain barrier as a function for sleep.
[See Original Abstract on Pubmed]
or technically,
Endocytosis at the Drosophila blood-brain barrier as a function for sleep.
[See Original Abstract on Pubmed]
Authors of the study: Gregory Artiushin, Shirley L Zhang, Hervé Tricoire, Amita Sehgal
Although neuronsA nerve cell that uses electrical and chemical signals to send information to other cells including other neurons and muscles are important in mediating sleep, the non-neuronal support cells of the brainThe brain is an organ that serves as the center of the nervous system in all vertebrate and most invertebrate animals. (known as glial cells) have also been linked to sleep regulation. Glial cells are a class of cells that surround all neuronsA nerve cell that uses electrical and chemical signals to send information to other cells including other neurons and muscles and are critical for their survival; they perform important ‘maintenance’ tasks for neuronsA nerve cell that uses electrical and chemical signals to send information to other cells including other neurons and muscles including providing them with nutrients and oxygen, insulating their electrical connections, and clearing dead cells and waste from their surroundings. Glial cells may help with waste clearance in the brainThe brain is an organ that serves as the center of the nervous system in all vertebrate and most invertebrate animals. during sleep, and can also release molecules that promote sleep2. In order to carry out their functions properly, glial cells have to move cargo into and out of the cell. This is mainly done through endocytosis, where things outside of the cell are captured into sacs and brought into the cell, much like packaging something important for transport. Although this process of endocytosis is important for glial cell performance overall, scientists still aren’t sure if endocytosis in glial cells is important for sleep. Additionally, it is not known which of the many types of glial cells are important in regulating sleep (there are over four main classes of glia).
Greg decided to use fruit flies to study the importance of endocytosis in sleep. Yes, flies sleep too! Not only are their sleeping patterns similar to that of humans, with a long period of sleep at night, but their genesA unit of DNA that encodes a protein and tells a cell how to function can also be easily manipulated in order to help scientists establish which genesA unit of DNA that encodes a protein and tells a cell how to function are important in regulating sleep. About 75% of known human disease genesA unit of DNA that encodes a protein and tells a cell how to function have a recognizable match in the genetic code of fruit flies3. These qualities make them a popular ‘model’ amongst scientists for studying sleep and its underlying mechanisms. To understand how endocytosis changed with increased sleep need, Greg deprived flies of sleep and then looked at how endocytosis was affected in their glial cells. He found that endocytosis was increased after sleep deprivation, and that this correlated with how sleep-deprived the flies were. Since this suggested that endocytosis was somehow linked to sleep, Greg wanted to explore this link further by blocking endocytosis entirely and seeing what happened to sleep. To do this, he generated a mutated form of a geneA unit of DNA that encodes a protein and tells a cell how to function that is critical for endocytosis in flies, allowing him to effectively block endocytosis in these animals. By mutating this geneA unit of DNA that encodes a protein and tells a cell how to function only in glial cells, Greg was able to block endocytosis exclusively in glial cells of the brainThe brain is an organ that serves as the center of the nervous system in all vertebrate and most invertebrate animals.. Interestingly, he saw that this increased how long the flies slept, suggesting that endocytosis in glia somehow controls the process of sleep.
Since there are many types of glial cells, Greg wanted to next understand which type of glial cells were important in sleep. Using the same genetic mutation strategy, Greg blocked endocytosis in each specific type of glial cell: he expressed the mutation in one type of glial cell at a time while leaving endocytosis in all of the other types of glia intact. This allowed him to determine which type of glial cell(s) was responsible for the effects he saw when he blocked endocytosis in all glial cells. He found that endocytosis in one particular type of glial cell was linked to sleep duration. This type of glial cell makes up the blood-brainThe brain is an organ that serves as the center of the nervous system in all vertebrate and most invertebrate animals. barrier in flies. The blood-brainThe brain is an organ that serves as the center of the nervous system in all vertebrate and most invertebrate animals. barrier, or BBB, is composed of tightly-linked glial cells that separate the brainThe brain is an organ that serves as the center of the nervous system in all vertebrate and most invertebrate animals. from the rest of the body. This barrier acts as a roadblock that prevents many substances from getting into the brainThe brain is an organ that serves as the center of the nervous system in all vertebrate and most invertebrate animals., which is crucial for protecting the brainThe brain is an organ that serves as the center of the nervous system in all vertebrate and most invertebrate animals. from pathogens or toxins. Greg found that blocking endocytosis only in the BBB glial cells caused changes in the structure of the barrier and increased sleep. However, blocking endocytosis in other types of glia did not affect the BBB or sleep.
Greg’s work suggests that endocytosis in the glial cells of the BBB of the fly is an important regulator of sleep, identifying a specific mechanism that may also be crucial in human sleep. Exactly how endocytosis at the BBB affects sleep duration remains unknown, but it is possible that this process may be important in waste clearance or maintenance of the BBB. If endocytosis is disrupted, these processes may be impaired leading us to sleep longer as a way of compensating. Future studies will aim to address why disrupting endocytosis in these BBB glial cells messes up the sleep cycle. Greg’s findings in this study (and future experiments) are important because they allow researchers to understand exactly how these processes at the BBB could be important for human brainThe brain is an organ that serves as the center of the nervous system in all vertebrate and most invertebrate animals. function, and how they may be altered in sleep deprivation and sleep disorders, such as insomnia. Scientists might finally be on track to figure out why pulling an all-nighter turns us into sleep-deprived zombies!
About the brief writer: Claudia Lopez
Claudia is a fourth year Neuroscience graduate student studying HIV-related neurodegeneration. She uses cell culture system to study how HIV infection leads to neuronal dysfunction.
Citations:
Shokri-Kojori E, Wang G, Wier CE, Demiral SB, Guo M, Kim SW . . . Volkow ND. (2018). β-Amyloid accumulation in the human brain after one night of sleep deprivation. Proceedings of the National Academy of Sciences, 115(17): 4483-4488. You can find the paper here.
Halassa MM, Florian C, Fellin T, Munoz JR, Lee S, Abel T . . . Frank MG. (2009). Astrocytic modulation of sleep homeostasis and cognitive consequences of sleep loss. Neuron, 61(2): 213-219. You can find the paper here.
Pandey UB & Nichols CD. (2001). Human disease models in Drosophila melanogaster and the role of the fly in therapeutic drug discovery. Pharmacological Reviews, 11(6): 1114-1125. You can find the paper here.