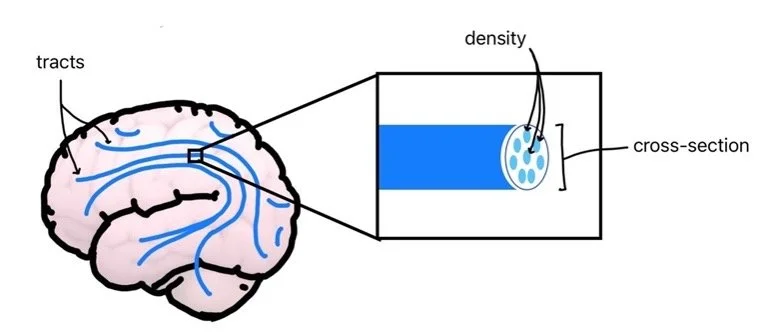
BRAINS IN BRIEFS
Scroll down to see new briefs about recent scientific publications by neuroscience graduate students at the University of Pennsylvania. Or search for your interests by key terms below (i.e. sleep, Alzheimer’s, autism).
A new discovery for how DNA unwinds in neurons to support brain health and memory
or technically,
Histone variant H2BE enhances chromatin accessibility in neurons to promote synaptic gene expression and long-term memory
[See original abstract on Pubmed]
Emily Feierman was the lead author on this study. Emily is currently a postdoc in the labs of Kiran Musunuru and Becca Ahrens-Nicklas at Penn/CHOP developing gene editing therapies for rare pediatric metabolic disorders.
or technically,
Histone variant H2BE enhances chromatin accessibility in neurons to promote synaptic gene expression and long-term memory
[See Original Abstract on Pubmed]
Authors of the study: Emily R Feierman, Sean Louzon, Nicholas A Prescott, Tracy Biaco, Qingzeng Gao, Qi Qiu, Kyuhyun Choi, Katherine C Palozola, Anna J Voss, Shreya D Mehta, Camille N Quaye, Katherine T Lynch, Marc V Fucillo, Hao Wu, Yael David, Erica Korb
While most of us know about the famous double-stranded structure of DNA, what is much less common knowledge is how DNA is stored inside of our cells. Think about it… a single cell in your body has so much DNA that if you laid it out, it would be almost 2 meters (or 6 and a half feet) long! In a flash back to biology class, you may remember that DNA is stored in 23 pairs of chromosomes in each cell which look like big fuzzy ‘X’s. And as it turns out, each of those chromosomes is really made up of DNA wrapped around lots of big blocky proteins called histones, almost like string wound around a bunch of yo-yos and stacked next to each other.
When a cell wants to read a gene from the DNA to make a new protein, it uses different mechanisms to partially unwind those histone yo-yos.This type of control (opening and closing access to DNA without changing the DNA itself) is called epigenetics. It helps cells decide which genes to use and when. This is especially important in the brain, where neurons need to turn on certain genes for learning, communication, and memory. Epigenetics is a very hot field of research because when this regulation goes wrong, cells may activate the wrong genes or fail to activate the right ones, which can contribute to neurological disease.
Emily Feierman, a recent NGG graduate, and her lab wanted to know how epigenetics plays a role in the brain – specifically how different histones can modify how tightly packed the DNA is in neurons across the brain and whether or not this can affect thinking and memory. To do this, she looked at a newly discovered component of the ‘yo-yos’, a histone protein called H2BE.
Before we talk more about H2BE we have to know a bit more about histones. Each ‘yo-yo’ is made up of four different histones which are usually standard. These are called H2A, H2B, H3, and H4. But it turns out, your cells can actually substitute in some less common histones to loosen up the DNA and allow different genes to be expressed. Each of the 4 standard histones can come in those less common flavors. While previous studies had found these flavor variants in the brain for H2A and H3, nobody had yet found any widely present flavors of the standard H2B histone building block.
Emily and her team were interested in ‘E’ variant of H2B specifically because over a decade ago H2BE had been shown to be highly present in neurons of the olfactory cortex of the brain, the area responsible for your ability to smell, but it wasn’t clear at the time whether or not it was present in the rest of the brain or what exactly it was doing.
To remedy this, Emily developed a new way to mark the presence of H2BE in neurons and found that instead of existing only in the olfactory area, it can actually be found in neurons all across the cortex of the brain. This suggests that it plays an important and more general role in supporting neurons since it’s found everywhere across the brain instead of just one area. She also found that H2BE loosens up the DNA strands more than normal H2B, so that genes can be more easily read out for protein production. But what proteins does H2BE help free up for production you may ask?
To answer this question, Emily used a mouse model gene knockout (KO) of H2BE, meaning the mice could not produce H2BE, and harvested neurons from their brains and compared the range of genes turned on in these mice relative to normal mice. She found that neurons from H2BE KO mice had changes in genes related to the functioning of neuronal synapses, the sites of contact between neurons that allow them to communicate.
One thing to know about synapses is that they are complicated button-like structures on the branches of neurons that require a lot of maintenance. Like little machines, they require scaffolding to build and molecular gizmos to make work, which requires a lot of different proteins to be made and therefore genes to be active. So any changes in synaptic gene expression in the H2BE KO mice might lead to impaired synaptic functions. When Emily tested this, this is exactly what she found - neurons from H2BE KO mice had reduced synaptic responses to electrical inputs.
It is also well known that the ‘strength’ of synapses and their density in neurons can play a role in memory formation. To test if H2BE plays a role in memory, Emily subjected the H2BE KO mice that have the deficits in synaptic functioning to different behavioral and memory tasks. While they didn’t seem to have any problems with their sense of smell, social activity, or anxiety, they did have reduced long term memory!
So it seems that by epigenetically controlling the looseness of DNA at key sites related to proteins used for synaptic functioning, the histone H2BE variant plays an important role in maintaining neuronal health and memory formation. Emily’s work is the first to fully characterize the role of this histone variant in brain functioning, and it opens up new insights into how gene expression is controlled in neurons to ultimately contribute to behavior, and how it could go wrong in aging and disease.
About the brief writer: Joe Stucynski
Joe is a graduate student in Dr. Franz Weber’s and Dr. Shinjae Chung’s labs at Penn. He is interested in how the immune system influences sleep regulation during sickness via interoceptive pathways.
Want to learn more about H2BE and epigenetics? You can find Emily’s full paper here!
How psychedelics remap the brain to help overcome traumatic fear
or technically,
Psilocybin-enhanced fear extinction linked to bidirectional modulation of cortical ensembles
[See original abstract on Pubmed]
Sophie A. Rogers was the lead author on this study. Sophie is a sixth-year PhD candidate investigating the impact of psychedelics on cortical computations underlying persistent fear and pain in Dr. Gregory Corder's laboratory at the University of Pennsylvania. Sophie's thesis work has been published in Nature Neuroscience, accepted in Nature, and was awarded an F31 training grant from the National Institute of Neurological Disorders and Stroke. In 2020, Sophie graduated from the University of Chicago with an Honors B.S. in Neuroscience, after completing an undergraduate thesis in the laboratory of Dr. Ming Xu. In 2026, she will join the laboratory of Dr. Maria Geffen at UPenn as a postdoctoral fellow studying the effect of psilocybin on auditory predictive processing in mouse models of autism spectrum disorder. In the future, Sophie hopes to become an independent academic researcher using large-scale neural recordings and computational techniques to understand how psychedelics alter mood, motivation, and decision-making across disease models.
or technically,
Psilocybin-enhanced fear extinction linked to bidirectional modulation of cortical ensembles
[See Original Abstract on Pubmed]
Authors of the study: Sophie A. Rogers, Elizabeth A. Heller & Gregory Corder
According to the World Health Organization, approximately 70% of people worldwide will experience a potentially traumatic event in their lifetime. Of these people, 5.6% will go on to develop post-traumatic stress disorder (PTSD), which is a condition where individuals have persistent, frightening thoughts and memories of the traumatic event. Often, individuals with PTSD experience sleep problems, feel detached or numb, or may be easily startled. Psychedelic drugs are being increasingly researched as a tool to help people overcome mental health conditions like PTSD. One of the most commonly studied drugs, and the focus of today's post, is called psilocybin. Although no large-scale clinical study has investigated psilocybin as a treatment for PTSD, scientists have found that even a single dose of psilocybin can have lasting impact on patients suffering from other neuropsychiatric disorders such as depression, substance use disorder and anxiety. In order to explore the effects of psilocybin on trauma, Sophie Rogers, a former NGG graduate student in Greg Corder’s lab, carried out experiments in mice to identify how psilocybin affects the brain after a traumatic events.
To begin exploring the question of how psilocybin produces its therapeutic effects on the brain, Sophie first needed to choose a way to model a traumatic event in mice. She used a very common experimental approach called trace fear conditioning (TFC). Under this approach, mice undergo a 5-day long experiment (Figure 1). On the first day, called habituation, mice are placed in a box (Box A) and a sound is played. The mice explore the environment and become familiar and comfortable with the box and the sound that is played. On the second day, mice are placed in a new box (Box B) and now, 20 seconds after the sound is played (the same sound from day 1), the mice receive a ~2 second electric shock, which is considered a traumatic event for the mice. On this day of the experiment, called acquisition, the mice learn to associate the sound played and the box environment with a painful memory. On the third, fourth and fifth days, the mice are returned to the original box (Box A) and the same sound is again played but without any shocks delivered. These final three days are called extinction, where the mice slowly learn that the sound no longer predicts the shock.
Figure 1. Illustration of the experimental approach used to investigate the effect of psilocybin on traumatic fear memories. The graphs below each box represent the relative amount of freezing that mice, on average, exhibited on a given day of the protocol.
Throughout the experiment, scientists can measure how afraid of the sound the mice are by measuring the amount of time that the mice spend freezing, defined as the complete absence of movement except for breathing. In this experiment, freezing levels are very low or zero on day 1 (habitation) but after the shock is delivered on day 2 (acquisition), the mice start to freeze for as high as 50% of the time. On day 3, the mice show a very high freezing response to the sound despite being in Box A, rather than Box B, where the shock took place. This demonstrates the fear memory of the mice is generalized to different contexts, which occurs similarly in PTSD patients. Throughout extinction (days 3-5), most mice show a decline in freezing behavior, suggesting that they are beginning to loosen the association between the sound and the shock. However, their overall freezing levels are still way higher than during the habitation day (before the shock was introduced at all), suggesting that the fear memory is still present.
Sophie next wanted to test whether giving mice the psilocybin drug directly after acquisition (day 2) affected how they responded during the extinction (days 3-5). If psilocybin helps in overcoming the traumatic memory, we would expect that psilocybin-treated mice would show even lower freezing on the extinction days than those that were not given psilocybin (control). Interestingly, Sophie found some mice were greatly benefited by the psilocybin, showing a large reduction in freezing compared to the control mice, but not all the mice that received psilocybin after acquisition responded this way. This suggests that there is significant individual variability when it comes to psilocybin’s effect on treating traumatic events. Nevertheless, Sopie wanted to take a closer look at the brains of the mice to determine if something was different between the group of mice that were benefitted by psilocybin versus the group that was not benefitted, along with the control group.
In order to determine how the psilocybin affected the brains of these mice after trace fear conditioning (TFC), Sophie measured the “activity” of single brain cells, or neurons, in an area called the retrosplenial cortex (RSC). Some neurons become active in response to things in our environment that they represent, for example a “sound neuron” might be activated by a sound being played. Other neurons become active in response to more internal things, like fear and memories. Sophie chose to focus on RSC because the neurons in the area are involved in storing and retrieving memories of lived experiences and because these neurons are involved in extinguishing fear memories. Sophie measured the activity of individual neurons in this brain area across the 5 days of trace fear conditioning (TFC). She found that in control mice, there was a group of neurons in the RSC that were strongly activated after the acquisition of the fear memory (day 2). During extinction (days 3-5) and while the control mice were freezing, this group of ‘fear neurons’ was highly active and did not vary much. This suggests that the fear neurons in RSC were inflexible while the mice remained focused on the strong association formed between the sound and the shock, even in the absence of the shock. When Sophie looked at the RSC neurons of mice that were given psilocybin and showed reduced freezing, she found that the group of fear neurons were less active during freezing and instead became more active in response to other things, like walking around. In other words, the neurons in the psilocybin mice were more flexible and able to dissociate from the original fear response. So Sophie found that psilocybin helps overcome traumatic experiences like TFC by allowing the brain to be more flexible, which enables therapeutic-like responses.
Taken together, Sophie’s results offer strong motivation for future studies of psilocybin on the treatment of neuropsychiatric disorders, like PTSD. Her work in mice shows us that psilocybin has a powerful effect on the brain’s ability to process and overcome traumatic events by changing how our brain responds to things associated with bad experiences. Furthermore, her finding that the effects of psilocybin can vary between individual mice leaves open the question of why some people respond differently to the same treatment. One possibility is that the mice that weren’t helped by psilocybin were already “behaviorally rigid” meaning that they were already prone to repetitive, inflexible behaviors. This may correspond to a person who suffers from PTSD, in addition to other neuropsychiatric disorders, like obsessive compulsive disorder, for example. It may be the case, therefore, that patients with multiple or overlapping disorders would not benefit from psilocybin treatment. Nevertheless, Sophie’s work provides invaluable insight into how psilocybin could be used as a therapeutic drug for the treatment of neuropsychiatric disorders, like PTSD.
About the brief writer: Jafar Bhatti
Jafar Bhatti is a PhD Candidate in the lab of Dr. Long Ding / Dr. Josh Gold. He is broadly interested in brain systems involved in sensory decision-making.
Want to dive deeper into how psilocybin affects the brain after trauma?
Check out Sophie’s full research paper here.
Changes in how brain regions “talk” to each other from childhood to adulthood follow and support a fundamental organizational pattern of the brain
or technically,
Functional connectivity development along the sensorimotor-association axis enhances the cortical hierarchy
[See original abstract on Pubmed]
Audrey Luo was the lead author on this study. Audrey is a 7th year MD/PhD student fascinated by brain development, which happens to be the topic of her graduate work with Ted Satterthwaite. Having recently defended her PhD, Audrey is heading back to the wards to finish her medical degree. She plans to pursue psychiatry and hopes to combine her research with clinical practice to better understand the human brain and treat mental illness.
or technically,
Functional connectivity development along the sensorimotor-association axis enhances the cortical hierarchy
[See Original Abstract on Pubmed]
Authors of the study: Audrey Luo, Valerie Sydnor, Adam Pines, Bart Larsen, Aaron Alexander-Bloch, Matthew Cieslak, Sydney Covitz, Andrew Chen, Nathalia Bianchini Esper, Eric Feczko, Alexandre Franco, Raquel Gur, Ruben Gur, Audrey Houghton, Fengling Hu, Arielle Keller, Gregory Kiar, Kahini Mehta, Giovanni Salum, Tinashe Tapera, Ting Xu, Chenying Zhao, Taylor Salo, Damien Fair, Russel Shinohara, Michael Milham, Ted Satterthwaite
The human brain supports a wide range of functions. Different regions of the outermost layer of the brain, the cortical surface, support two categories of functions—sensorimotor regions are involved in sensation and movement and association regions are involved in functions like self-reflection and emotion.
However, these different cortical regions can be distinguished from each other further beyond these two broad categories. We can use a framework known as the sensorimotor-association (S-A) axis. Instead of just categorizing each region as either a sensorimotor or association region, the S-A axis ranks the different brain regions from a sensorimotor end to an association end. Think of this as the difference between simply categorizing people as either “Gen Z” or “Millenial” versus ranking them from youngest to oldest— the ranking method is much more useful for meaningfully distinguishing between people!
Previous studies have shown that the S-A axis summarizes how many characteristics, including the brain’s physical structure, blood flow, and cell type composition, differ across the cortical surface. Because of the prevalence of this cortical surface pattern across so many biological brain measures, neuroscientists believe that the S-A axis is essential to the human brain’s ability to carry out its wide variety of functions.
In this paper, NGG student Audrey Luo and her colleagues in the Satterthwaite lab were interested in how different parts of the brain communicate with each other during the transition from childhood to adulthood. Imagine each brain region has a nonstop video call link that any other region can join at any time. When two regions’ activities are synchronized, it’s as if they have connected on video, communicating while their “screens” are in sync—neuroscientists call that functional connectivity (FC). Audrey wanted to know how the change in functional connections between different cortical regions during development related to the S-A axis. To do this, Audrey used data from participants ages 5 to 23 scanned with functional magnetic resonance imaging (fMRI). fMRI is an imaging research tool that can tell us when different areas of the brain are getting oxygen-rich blood, which roughly reflects which parts of the brain are activated.
Because Audrey included 200 cortical regions in her analysis, there could be an astonishing 19,900 possible FC pairs, or 19,900 “video calls”. To summarize across all these connections, Audrey gave each region a single score called FC strength—the average of a region’s connectivity to all the rest of the cortical regions. A high score means the region’s activity tends to stay in sync with many other regions (e.g., long calls with many partners, shared screen, very social); a low score means its activity tends to differ from other regions (e.g., short calls with few partners, distinct screen, less social). With this one-number summary of FC strength, Audrey tracked how each region’s connectivity to the rest of the brain changed from childhood to adulthood to ask the question: how is FC strength development across the cortical surface related to the S-A axis?
What Audrey found was that as children grew older, sensorimotor regions showed increased connectivity and association regions showed decreased connectivity. More specifically, Audrey showed that the pattern of change in FC strength of each brain region was directly related to its S-A axis rank: the closer the brain region was to the “sensorimotor end” of the axis, the more likely it was to increase in connectivity (i.e., more social), and the closer the brain region was to the “association end” of the axis, the more likely it was to decrease in connectivity (i.e., less social). In other words, functional connections don’t develop uniformly across the cortical surface. Instead, they follow a specific pattern that matches the fundamental organizational framework of the S-A axis.
To further understand how functional connectivity during development related to the S-A axis, Audrey asked whether the change in FC between a pair of regions depends on their identities as either sensorimotor or association regions. Bringing back the video call analogy, we can think of sensorimotor and association regions as two friend groups. Essentially, Audrey is asking—how does video call frequency between and within the two friend groups change over development? What she found was that as children grew into adults, connectivity between sensorimotor regions increased, connectivity between association regions decreased, and connectivity between association and sensorimotor regions also decreased. This paints a more specific picture of how functional connectivity develops: sensorimotor regions become more social (but just with each other), while association areas become more independent and specialized.
Overall, this work suggests that the S-A axis is not only a framework of how the brain is organized in adulthood, but also how the brain develops from childhood onward. Audrey argues that this specific pattern of changes in how sensorimotor and association areas communicate ultimately helps establish the S-A axis as the dominant cortical framework of the brain, enabling the vast diversity of functions observed in adulthood.
Perhaps the most impressive aspect of the paper is it reports the same results from not just one dataset, but four large datasets that were collected from different sites and in different ways. Indeed, some of Audrey’s findings are replications of results found in a paper by a graduated NGG student, Adam Pines: https://www.upennglia.com/briefs/bib-personalized-brain-networks— an impressive study conducted in one large dataset. Now, with Audrey’s rigorous work in four large datasets, we can have even more confidence as a scientific community that these findings on the S-A axis are not just dependent on the specific circumstances of a particular sample, but instead, capture something real about human biology. This is a concept known as generalizability—the extent to which findings can be applied to the entire population beyond a specific sample. This study is an excellent example of generalizable science and reveals a rigorous framework of functional brain development. Hats off to Audrey and the rest of the team!
About the brief writer: Kevin Sun
Kevin is co-mentored by Drs. Aaron Alexander-Bloch and Ted Satterthwaite, working with functional neuroimaging data to ask questions about development, genetics, and transdiagnostic psychopathology risk. Outside of research, Kevin enjoys film, weightlifting, art museums, and speculative fiction.
If you’d like to read more, see the original paper here!
Not all neurons shake during seizures
or technically,
Ndnf Interneuron Excitability Is Spared in a Mouse Model of Dravet Syndrome
[See original abstract on Pubmed]
Sophie Liebergalls is a 7th MD-PhD student who is mentored by Ethan Goldberg at the Children's Hospital of Philadelphia. She is interested in how different types of neurons in the brain send signals to other neurons along their axons, and how this process may be impaired in diseases like epilepsy. After completing medical school, she plans to do a residency in Pediatric Neurology and try to bring insights from the lab to the care of children with neurologic diseases.
or technically,
Ndnf Interneuron Excitability Is Spared in a Mouse Model of Dravet Syndrome
[See Original Abstract on Pubmed]
Authors of the study: Sophie Liebergall, Ethan Goldberg.
Neurons, the cells in our brains, communicate with each other with electricity. Usually, the amount of electricity is carefully regulated, allowing neurons to pass signals to each other in a controlled way. However, sometimes there is a sudden burst of electricity in the brain, causing a seizure. Many people will experience a single seizure during their lifetime, but people with regular seizures are diagnosed with epilepsy. Epilepsy is a broad category of brain disease that affects up to 50 million people worldwide. Some types of epilepsy can be managed with medication, but others are treatment-resistant and very difficult to control.
Dravet syndrome is one example of a disorder that causes treatment-resistant epilepsy. It’s a rare neurodevelopmental disorder that causes severe, frequent seizures starting in the first year of life, plus intellectual disability and developmental delay. Scientists have figured out what causes this disorder: mutations in a single gene called SCN1A. SCN1A is a gene that contains the instructions to make Nav1.1, one of the brain’s sodium channels. Some types of neurons in the brain rely on Nav1.1 to generate and control their electrical signals. Even though we understand the cause, there is still no cure for Dravet syndrome, and there is no current treatment that can fully manage patients’ symptoms. Scientists are still working to better understand the effects of mutations in SCN1A in neurons, with the hopes that new knowledge could eventually lead to new treatment strategies.
There are many different types of neurons in the brain, and scientists have learned that the gene SCN1A is most highly expressed in a group of neurons called inhibitory neurons. These neurons work by quieting their neighbors, helping to prevent runaway activity in the brain. Because SCN1A is especially important for these inhibitory neurons, scientists think that mutations in this gene disrupt the brain’s electrical balance: when inhibitory neurons can’t do their job, their neighbors can become overactive, leading to seizures.
Sophie Liebergall, a current NGG student, wanted to learn more about whether all inhibitory neurons are affected by SCN1A mutations. Specifically, she looked at a group of inhibitory neurons that express a protein called Ndnf. SCN1A mutations, like those that cause Dravet syndrome, impair the ability of some types of inhibitory neurons to generate electrical signals, but we didn’t know anything about what happens to Ndnf inhibitory neurons. The goal of Sophie’s study was to see whether Ndnf inhibitory neurons are disrupted similarly to other inhibitory neurons when there are errors in the SCN1A gene.
To investigate this question, Sophie used a mouse model of Dravet syndrome, where one of the two copies of Scn1a was genetically removed from all neurons. She then measured the electrical properties and activity of Ndnf inhibitory neurons in these mice, and she compared their properties to Ndnf interneurons in healthy mice with both working copies of Scn1a.
Surprisingly, Sophie found that Ndnf inhibitory neurons seemed totally unaffected by the missing copy of Scn1a -- their electrical properties were similar to those of healthy neurons. This was unexpected because the electrical properties of other types of inhibitory neurons are really different when they’re missing a copy of Scn1a. Sophie also found that these neurons do not seem to depend on the protein encoded by Scn1a to generate their electrical signals, which may explain why their activity was normal.
Sophie’s findings are important because they challenge the simple version of the hypothesis that seizures in Dravet syndrome are caused by a blanket loss of inhibitory neuron function. Instead, Sophie’s study shows that the story is more complex: some inhibitory neurons, like Ndnf neurons, remain fully functional even when Scn1a is missing. These results reshape our understanding of the disease mechanisms of Dravet syndrome and open the door to more targeted approaches in treating the disorder. More broadly, Sophie’s work reminds us to pay attention to the full diversity of neurons. The brain is complex, and it is only by appreciating and dissecting this complexity that we will be able to understand how the brain works and how it goes wrong.
Great work Sophie!
About the brief writer: Lyndsay Hastings
Lyndsay Hastings is a PhD candidate in NGG working in Dr. Tim Machado’s lab. She is interested in how the brain generates flexible and complex movement.
Curious about Sophie’s research? Check out the full paper here!
How Brain Injuries Affect Sleep-Related Neurons Differently in Male and Female Mice
or technically,
Mild Traumatic Brain Injury Affects Orexin/Hypocretin Physiology Differently in Male and Female Mice
[See original abstract on Pubmed]
Rebecca Somach was the lead author on this study in the Cohen lab. Rebecca is currently doing her postdoctoral fellowship at Swarthmore College. Her current research uses planarians as a model system to understand how pesticides affect developmental neurotoxicity. She's interested in helping students understand neurobiology and helping them achieve their research goals.
or technically,
Mild Traumatic Brain Injury Affects Orexin/Hypocretin Physiology Differently in Male and Female Mice
[See Original Abstract on Pubmed]
Authors of the study: Rebecca T Somach, Ian D Jean, Anthony M Farrugia, Akiva S Cohen
A traumatic brain injury (TBI) occurs when an external source, such as a strong hit to the head, damages the brain. TBIs can be mild or severe, with mild TBIs (mTBI) being especially common and accounting for a large portion of TBI cases. Both TBI and mTBI can negatively impact brain physiology and lead to problems like sleep issues. Surprisingly, despite a large portion of TBI patients experiencing disordered sleep, there is very little research focusing on how these injuries may affect neural circuits that regulate sleep. Understanding the link between TBI and disrupted sleep is an important step in improving treatments for TBI patients.
Orexin/hypocretin neurons are specialized brain cells found in a brain region called the Lateral Hypothalamus. These neurons play a critical role in sleep and wake regulation. When they are not working properly or if they die off, it can lead to sleep-related issues, such as narcolepsy (a disorder where people fall asleep unexpectedly) or excessive sleepiness during the day. Some research suggests that TBIs can disrupt orexin, which could explain why many TBI patients have sleep troubles.
However, the Cohen lab found that sleep problems after mTBI can happen even when the number of orexin neurons does not decrease due to the injury. This raised an interesting and important question: is it the activity of these neurons after injury, rather than how many there are overall, that is important? To tackle this question, the Cohen lab looked at how active orexin neurons were after mTBI by labeling them for cFOS, a protein that is produced when neurons are active. They found that instead of orexin neurons dying off, the activity of the overall population was actually reduced (less cFOS in the population), suggesting that the number of neurons alone is not the only factor to consider after TBI. These findings were important, as only two other studies have looked at how orexin neuron activity changes after brain injury.
Rebecca Somach, an NGG graduate from the Cohen lab, decided to further expand on this work by incorporating other fascinating neuroscience techniques. These included electrophysiological recordings, which measure electrical activity in the brain, and a mouse that has orexin neurons tagged with a glowing protein, making it easier to study and see these neurons in the brain. Rebecca also wanted to examine the effects of brain injury might be different in male and female mice, since sleep can vary between sexes. This is particularly important because of the little research on the effects of TBI, specifically mTBI, on orexin in female animals.
Rebecca specifically examined whether mTBI changes how orexin neurons might respond to electrical signals or how often they fire in male and female mice. To do this, they used current clamp recording, a neuroscience technique used to see how neurons react when they are injected with electrical current. In female mice, they found that orexin neurons became more negatively charged after mTBI, which is a process called hyperpolarization. In contrast, they found that in male mice, orexin neurons had a reduction in action potential threshold after mTBI, making it easier for them to fire. These seemingly small changes in how neurons behave after mTBI can have a big impact on how they communicate to other areas of the brain.
While orexin neurons are found in the Lateral Hypothalamus and talk to neurons adjacent to them, they are also connected to many different parts of the brain. Because of this, Rebecca wanted to examine whether mTBI could affect the connections these neurons receive from other brain regions. Orexin neurons receive both excitatory and inhibitory signals. To only focus on excitatory signals, Rebecca recorded neurons while applying a chemical called bicuculline, that blocks inhibitory signals. This allowed them to study how only excitatory signals may have changed after brain injury in male and female mice. They looked at two types of excitatory post-synaptic currents - spontaneous (sEPSC) and miniature (mEPSC), which can be thought of as the orexin neurons’ responses to excitatory inputs from other neurons. In short, the stronger the EPSCs, the more likely the neurons are to become active. After injury, they saw that these signals were less frequent and weaker in both male and female mice, meaning that the orexin neurons were getting less excitatory activity.
Next, Rebecca did the reverse experiment and isolated only the inhibitory signals to examine if there were mTBI-induced changes. She did this by recording orexin neurons with AP5 and CNQX, which are chemicals that remove excitatory currents. Rather than looking at sEPSCs and mESPSCs, she then focused on recording spontaneous and miniature inhibitory currents (sIPSC, mIPSC) in male and female mice. After injury, she saw reduced time between sIPSCs, meaning they occurred more often. Interestingly, she only saw stronger sIPSCs and mIPSCs in female mice, which could help explain why sleep disturbances after TBI can vary depending on sex.
Overall, while previous research has shown an effect of mTBIs on orexin neurons, not many studies looked at how brain injury impacts the activity of these neurons. Rebecca addressed this gap by exploring how the activity and connections of orexin neurons are altered after brain injury in both males and females. She shows that mTBI significantly alters how orexin neurons respond to inputs from other neurons and that although these changes appear similar between male and female mice, the underlying mechanisms are not the same. Ultimately, her work provides insight into how brain injury may disrupt sleep-related circuits and informs us why TBI patients may experience disordered sleep.
About the brief writer: Diana Pham
Diana is a Neuroscience Ph.D. student in Dr. Brett Foster’s lab. She is broadly interested in how memories are encoded and consolidated in the brain.
Want to learn more about the effects of brain injury on sleep-related neurons? You can find Rebecca’s full paper here!
How does the brain transform our memories during sleep?
Or technically,
Memory reactivation during sleep does not act holistically on object memory
About the author: Liz Siefert
Liz is a 4th year PhD candidate working with Dr. Anna Schapiro and Dr. Brett Foster. She is interested in how our memories change and shift overtime, and the role of arousal states (wake, sleep) in these changes.
or technically,
Memory reactivation during sleep does not act holistically on object memory
[See Original Abstract on Pubmed]
Authors of the study: Elizabeth M. Siefert, Sindhuja Uppuluri, Jianing Mu, Marlie C. Tandoc, James W. Antony, Anna C. Schapiro
Whether you’re a human, dog, or fruit fly, we all sleep. There’s no doubt that sleep is essential, but why we sleep is still hotly debated. Many ideas have centered around the observation that sleep is important for strengthening key memories and putting them into storage. Neuroscientists believe this happens during sleep when the brain replays memories to strengthen them, a process called memory reactivation. Supporting this idea, numerous studies have shown that people tend to do better if they take even a short nap between learning something and being tested on it.
NGG student Elizabeth Siefert was fascinated by the process of memory reactivation during sleep, but wanted to better understand what impact it has on our memories. She noticed that sleep can’t just be improving memory overall, because that’s not how memory works. “As we go about our lives, our memories don’t always continue to improve,” says Siefert. “Often, they get worse, or they change in different ways. So really, across time, memory isn’t just improving, but it’s transforming.” Following on these observations, Siefert designed an experiment to understand whether sleep simply improves memory or rather has the power to transform it by strengthening some aspects of a memory while allowing others to fade.
Studying the sleeping brain
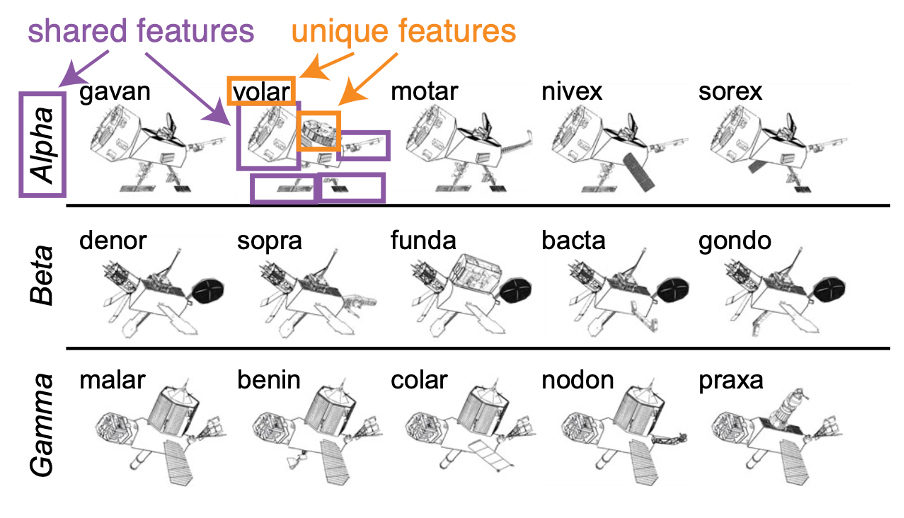
To probe the nature of memory transformations during sleep, Siefert and her team needed a memory test that allowed them to assess different aspects of memory. “Memories have lots of different features, so we wanted to know if memory reactivation has the power to act on different features of our memories in different ways,” says Siefert. She did this by asking participants to learn the identities of several satellites belonging to three groups. The satellites were created by mixing different parts so that the team could control the different features of a memory (Figure 1). Some shared features appeared on multiple satellites within a group, but never on satellites in other groups. Other unique features were specific to just one satellite, allowing the participants to identify it by name. This allowed the team to look at how representations of the individual versus the shared features were transformed in memory during sleep. Siefert asked the participants to learn the satellite names and groups, had them take a nap, and then tested how well they remembered the unique versus shared satellite features.
Just because participants took a nap doesn’t mean they were necessarily going to replay their memories of the satellites. That’s why Siefert stepped in to nudge their sleeping brains to replay the memories she was interested in. She did this using a method called targeted memory reactivation (TMR). During TMR, experimenters measure a participant’s brain activity while they sleep and look for moments when the sleeping brain is most likely to replay a memory. When those moments are identified, the experimenter plays a cue to remind them of a recent memory and encourage the brain to replay that specific memory. The cues are played softly enough that they don’t wake up the participant, and participants don’t know that the cues are being played, so any impacts of TMR are unconscious. “The best method we know in humans for studying memory reactivation in sleep is TMR,” says Siefert. “Our sleeping brain is already prioritizing certain information, and what we’re doing with TMR is biasing the brain to prioritize what we want it to.” In Siefert’s experiment, she played the name of some of the satellites the participant had just learned to encourage reactivation of those memories.
Figure 1. Satellites used in the memory task. Satellites belonged to three groups: alpha, beta, or gamma. The purple and orange boxes highlight the shared and unique features of the satellite volar. The purple boxes show volar’s features that are shared with other satellites in the alpha category, while the orange boxes show the features that are unique to volar.
Clarifying the relationship between sleep and memory
With all the pieces in place, Siefert was ready to ask how memory replay during sleep impacted different features of memory. Before the nap, participants tended to do a better job learning the satellites’ unique features compared to their shared features. In other words, they were better able to identify that a particular feature belonged to an individual satellite than that a particular feature was shared by satellites of a certain group. After a nap with TMR, that divide only widened. Siefert found that TMR increased participants’ memory for unique features while decreasing their memory for shared features. For satellites whose names were not used as cues during sleep, there was no impact on memory. This demonstrates that the effects were likely due to the reactivation encouraged by TMR. “That showed us that memory reactivation during sleep, it’s not just wholistically improving our memory, it has the power to act on specific features of our memory in very specific ways,” says Siefert. “It [can] even impact different features of our memory in different ways, such as improving some even at the cost of others.”
Why were certain features strengthened while others got weaker with reactivation? One possibility is that the cues used to trigger replay during sleep were the satellite names. This may have encouraged the brain to prioritize information about the individuals’ identity rather than their group membership. Future studies could use the group names as a cue instead and see if that nudges the brain to prioritize shared over unique features. Another possibility is the fact that people already tended to learn the unique features better before the nap. That prioritization may have carried over into their sleeping brains. “Your learning strategy and goals before sleep might bias what type of information the sleeping brain wants to reactivate,” says Siefert. “It’s possible that it was those learning strategies that led that information to be benefitted more by sleep.” Importantly, no matter what the explanation, it’s not the case that unique features will always be remembered over shared features following a night of sleep. Instead, a complex combination of learning goals and strategies likely shape exactly how memories are transformed during sleep.
Unbeknownst to the participants, Siefert was also testing how the way she presented the cues during sleep impacted memory. Sometimes she played the same satellite name over and over in a block, and other times she played satellite names intermixed with each other. Importantly, she always played the satellite names the same number of times during sleep, only changing in what order she played them. While both methods led to improved memory for unique over shared features, repeating the same satellite name many times in a row was more effective in transforming memory than playing the names in a random order. Siefert suggests that this may be evidence that reactivating memories in blocks helped the brain differentiate things in memory more than random reactivations.
The lab is already extending their results to understand more about the relationship between sleep and memory. Specifically, they’re interested in how sleep may help us take new information and incorporate it into older memories. “A study that we’ve run in the lab since [mine] is trying to understand what it looks like to learn new information that is aligned with things that you know from the past and how that new information can become integrated into older memories without totally overwhelming those old memories,” says Siefert. It seems that we’re only scratching the surface of what TMR can teach us about the sleeping brain’s relationship to memory.
What does this mean for everyday life?
After learning about these results, you might wonder whether students should give up on wakeful studying and just play their textbooks while they sleep. Unfortunately, it’s still not that easy. “Because [memory reactivation] doesn’t just improve memory and has this transformation component, if you took this device home and used it to play a textbook it’s not clear to me whether it would improve or hurt your memory of that information,” says Siefert. “You might need specific learning goals, you would need to think carefully about when you are delivering the cue, and you need to consider lots of other things.” It may be possible to design a system that could help a student study in their sleep, but we still need to learn a lot more about how TMR works before that will be possible.
Despite its complicated nature, some scientists are optimistic that they may be able to bring TMR to your bedroom. Rather than recording brain activity to target specific moments for reactivation, they aim to use audio recordings of movement during sleep to target the longer periods of sleep when they think replay naturally occurs. “For me in the lab it’s really important to target specific moments so I really know what my cues are doing, but in the real world that might be less important,” says Siefert. TMR is already being used in some clinical settings to help stroke patients relearn how to move their bodies, and Siefert suggested that it could one day be helpful for things like language learning if you’ve already learned some of the basics. For those who may be worried about TMR being used for brain washing or unconscious influence, Siefert says we aren’t capable of that now and may never be. “Your brain is already doing things and we’re just biasing it to little quick moments,” says Siefert. “We aren’t at the point where we can totally change the way that you’re thinking about something.”
Even in the absence of a TMR system on your nightstand, one takeaway is clear: sleep is important. “We don’t have a good understanding of why we’re remembering and forgetting certain things but knowing that the brain is selecting information means that that selection is probably important, and that selection is clearly happening during sleep,” says Siefert. “That means you should be getting good sleep, because you want to allow your brain time to process the information.” So next time you’re faced with the decision to stay up a little later studying for a test or prepping for a meeting, remember that choosing to sleep may be even more important than putting in that extra half hour of work.
About the brief writer: Catrina Hacker
Catrina Hacker is a PhD candidate working in Dr. Nicole Rust’s Lab. She is broadly interested in the neural correlates of cognitive processes and is currently studying how we remember what we see.
Learn more about the team’s memory reactivation study in the original paper.
A NEW APPROACH TO IMAGING THE BRAIN DURING EARLY-STAGE NEURODEGENERATION
or technically,
Positron Emission Tomography with [18F]ROStrace Reveals Progressive Elevations in Oxidative Stress in a Mouse Model of Alpha-Synucleinopathy
[See original abstract on Pubmed]
Evan Gallagher is a recent graduate of Penn’s neuroscience graduate program, and the lead author on this study. He is broadly interested in using neuroimaging approaches like magnetic resonance imaging (MRI) and positron emission tomography (PET) to study complex biological processes in living animals and people. Ultimately, he hopes that his work allows us to better understand—and eventually treat—major neurological disorders like Alzheimer’s and Parkinson’s.
or technically,
Positron emission tomography with [18F]ROStrace reveals progressive elevations in oxidative stress in a mouse model of alpha-synucleinopathy
[See Original Abstract on Pubmed]
Authors of the study: Evan Gallagher, Catherine Hou, Yi Zhu, Chia-Ju Hsieh, Hsiaoju Lee, Shihong Li, Kuiying Xu, Patrick Henderson, Rea Chroneos, Malkah Sheldon, Shaipreeah Riley, Kelvin C. Luk, Robert H. Mach and Meagan J. McManus
Neurodegenerative diseases, like Alzheimer’s and Parkinson’s, impact 15% of the world’s population [1]. This percentage has climbed considerably over the last 30 years and is expected to continue rising as the global population ages [2]. Still, the process of diagnosing (and therefore treating) neurodegenerative diseases remains quite challenging. The difficulty arises because brain changes that play a central role in the disease process begin years or even decades before people experience symptoms [3]. In other words, by the time patients notice physical changes (e.g., memory loss, difficulties with movement, etc.), their brain is already dramatically and permanently affected.
What if we had some way to detect and visualize very early neurodegeneration-related brain changes? Doctors could then identify people at risk for developing neurodegenerative diseases and intervene with treatments before things escalate beyond repair. This is the vision that inspired recent work by Neuroscience Graduate Group (NGG) alum Dr. Evan Gallagher in collaboration with the labs of Dr. Robert Mach and Dr. Meagan McManus at the University of Pennsylvania.
In order to find early signs of trouble, the team needed something that serves as a “red flag” for neurodegeneration as well as a way to find and measure it in the living brain. They landed on reactive oxygen species (ROS), which are molecules that exist naturally in the body, but that can lead to problems if they build up over time. “Excessive production of reactive oxygen species is an important, central process in many neurodegenerative diseases,” explains Dr. Gallagher. “It’s also a very early process. This means that if we’re able to detect and quantify reactive oxygen species in the brain, we may be able to identify that there’s a problem much earlier than we are currently doing.” To find and label reactive oxygen species in the brain, chemists in Dr. Bob Mach’s lab engineered a chemical called ROStrace [4]. ROStrace is a positron emission tomography (PET) imaging radiotracer. When injected into the body, ROStrace travels through the bloodstream and into the brain where it targets and tags reactive oxygen species, giving off a radioactive signal that can be detected. This means that researchers can perform a ROStrace PET imaging scan and get out an image of the brain that looks and works a lot like a heatmap with the color and pattern of the image relaying information about the number of reactive oxygen species present across brain regions. With these PET imaging heatmaps, researchers are able to find “hot spots” of highly-concentrated reactive oxygen species, which could be indicators of places in the brain where the groundwork for neurodegeneration is being laid.
To validate that their ROStrace technology works and prove that it can be used to detect early signs of neurodegeneration, the Mach and McManus labs performed a series of well-controlled experiments in a mouse model. In particular, they used a mouse model with a mutated form of the human alpha synuclein protein. The mutation causes alpha synuclein to accumulate as mice age, the same process that happens in a number of human neurodegenerative diseases. Dr. Gallagher performed ROStrace PET imaging on these animals when they were either 6 or 12 months old (middle and old age for this type of mouse) and compared the results to healthy mice in the same age groups. He found that the mutant alpha synuclein mice had higher levels of reactive oxygen species than the healthy animals at both timepoints. However, it wasn’t just the overall counts of reactive oxygen species that differed. Looking at the ROStrace brain images, Dr. Gallagher could identify a clear spatial pattern with which reactive oxygen species spread across the brain over time in the mutant alpha synuclein animals. It was the same pattern he found when he took brain tissue from mutant animals and stained it with special chemicals to label reactive oxygen species. In other words, it seemed like his PET scan brain images matched the reality of reactive oxygen spread as seen in mouse brains under the microscope. ROStrace worked! “It’s really exciting,” says Dr. Gallagher. “The fact that our PET images matched up with what we found in tissue means we actually have a way of detecting reactive oxygen species in the brains of living animals.”
Taking things one step further, Dr. Gallagher wanted to verify that the ROStrace signal was highlighting something biologically and clinically useful. That is to say, he wanted to confirm that the reactive oxygen species seen on ROStrace brain images were actually related to alpha synuclein disease pathology. Using brain tissue from the same mutant mouse species, this time he stained it with chemicals to mark reactive oxygen species and alpha synuclein proteins. Dr. Gallagher found that the two chemicals labeled many of the same brain cells and brain regions, suggesting that reactive oxygen species were, in fact, associated with alpha synuclein pathology. Remarkably, the co-occurrence of reactive oxygen species and alpha synuclein in the brain was detectable months before mice showed severe behavioral symptoms. This implies that the ROStrace signal could one day be used to diagnose -- or even to preemptively screen for -- alpha synuclein-related brain diseases, like Parkinson’s or Alzheimer’s, long before patients realize something is wrong.
ROStrace could also have applications beyond serving as a marker for alpha synuclein accumulation and neurodegeneration. “As far as we can tell, just about everything ‘bad’ happening in the body is associated with reactive oxygen species,” offers Dr. Gallagher. That means that the methods used in this study should be generalizable across many other disorders. This generalizability, however, does come with a downside. “One bad thing that could be happening in your body is alpha synuclein aggregation, but that’s certainly not going to be the only bad thing happening in your body at any given time,” Dr. Gallagher continues. “For instance, a stressful event or a bad night of sleep can cause increased levels of reactive oxygen species. ROStrace can pick up on that and it makes interpreting a single scan really tricky.” In the current study, Dr. Gallagher navigated this limitation by using scans from many mice to find differences between sick and healthy groups. However, being able to get interpretable information from one single scan is critically important when we think about using technologies, like ROStrace, in the clinic. So, while this study represents an exciting first step, for now research with ROStrace has been limited to lab animals and there are still hurdles to clear before human subjects are part of the picture.
About the brief writer: Kara McGaughey
Kara is a PhD candidate in Josh Gold’s lab studying how we make decisions in the face of uncertainty and instability. Combining electrophysiology and computational modeling, she’s investigating the neural mechanisms that may underlie this adaptive behavior.
Citations:
Van Schependom, J., & D’haeseleer, M. (2023). Advances in Neurodegenerative Diseases. Journal of Clinical Medicine, 12(5), 1709. https://doi.org/10.3390/jcm12051709
Brown, R. C., Lockwood, A. H., & Sonawane, B. R. (2005). Neurodegenerative Diseases: An Overview of Environmental Risk Factors. Environmental Health Perspectives, 113(9), 1250–1256. https://doi.org/10.1289/ehp.7567
Gallagher, E., Hou, C., Zhu, Y., Hsieh, C.-J., Lee, H., Li, S., Xu, K., Henderson, P., Chroneos, R., Sheldon, M., Riley, S., Luk, K. C., Mach, R. H., & McManus, M. J. (2024). Positron Emission Tomography with [18F]ROStrace Reveals Progressive Elevations in Oxidative Stress in a Mouse Model of Alpha-Synucleinopathy. International Journal of Molecular Sciences, 25(9), 4943. https://doi.org/10.3390/ijms25094943
Hou, C., Hsieh, C.-J., Li, S., Lee, H., Graham, T. J., Xu, K., Weng, C.-C., Doot, R. K., Chu, W., Chakraborty, S. K., Dugan, L. L., Mintun, M. A., & Mach, R. H. (2018). Development of a Positron Emission Tomography Radiotracer for Imaging Elevated Levels of Superoxide in Neuroinflammation. ACS Chemical Neuroscience, 9(3), 578–586. https://doi.org/10.1021/acschemneuro.7b00385
Interested in reading more about ROStrace? You can check out Evan’s paper Here!
How genes influence social behavior in animals
or technically,
Conserved autism-associated genes tune social feeding behavior in C. elegans
[See original abstract on Pubmed]
Mara Cowen was the lead author on this study. Mara was co-mentored by Dr. Mike Hart and Dr. David Raizen and researched the effect of mutations in the autism-related gene, Neurexin, on aggregation, stress response, sleep, and neuronal morphology in C. elegans as part of the Autism Spectrum Program of Excellence (ASPE). When not in the lab, Mara can be found traveling, baking, binge-watching Netflix, and going to breweries with her rescue Australian Shepherd, Crispr!
or technically,
Conserved autism-associated genes tune social feeding behavior in C. elegans
[See Original Abstract on Pubmed]
Authors of the study: Mara H. Cowen, Dustin Haskell, Kristi Zoga, Kirthi C. Reddy, Sreekanth H. Chalasani & Michael P. Hart
Most people, including myself, love having lunch with their friends. This kind of behavior is known as social feeding and is common across many animals including most species of birds, fish, mammals, and even insects. Social feeding, in addition to other behaviors that animals exhibit, is controlled by the nervous system which is made up of nerve cells called neurons. Despite the fact that different animal species have different nervous systems, social feeding occurs almost universally across the animal kingdom. This begs the question: what in the nervous system makes it such that animals prefer social feeding as compared to eating alone? Mara, a former NGG graduate student, and her lab, thought that perhaps this was due to changes in an individual’s genes - the blueprints for how the body should grow, develop, and function.
To explore this question of how genes might influence social feeding, Mara studied the behavior of an animal called C. elegans, which is a worm-like animal about the size of a grain of sand. Despite their small size and relatively simple nervous system, these worms can perform many different social behaviors, including social feeding. Social worms tend to clump up together, or aggregate, while eating. In addition to exhibiting the behavior that Mara is interested in, C. elegans are commonly used in labs because experimenters have very precise control over the genes that the worms express. Worms that express a particular set of genes are referred to as a strain. This makes C. elegans the perfect animal to study how genes influence social feeding behavior.
Mara began her study using two worm strains that show opposite social feeding behaviors - one strain that shows consistent social feeding, the social control strain (Figure 1A), and one strain that prefers to eat alone, the solitary control strain (Figure 1D). For both strains, she counted the number of worms that aggregated and the number of worms that did not. She found that 78% of the social control worms ate together, whereas only 4% of the solitary control worms ate together.
Figure 1. The effect of different genes on social feeding behavior in C. elegans.
Now that she has these two worm strains as reference points, Mara’s main goal was to understand how different genes affected social feeding behavior. However, most animals, including C. elegans, have tens of thousands of genes - how was Mara to know which genes would be involved in social feeding behavior? This is where she and her lab used insight from past studies of human social conditions, such as autism. Recent work studying autism found that the neurexin gene and the neuroligin gene were highly associated with autism. Traditionally, these two genes were known to only be involved in allowing neurons to connect with one another. More recent work exploring the function of these genes suggests that they also play a role in controlling social behavior. Therefore, Mara asked whether the neurexin and neuroligin genes could also be playing a role in social feeding in C. elegans and, if so, how. Indeed, when Mara disrupted the neuroligin or the neurexin gene in the social control strain, the worms displayed significantly less social feeding behavior (Figure 1B). Interestingly, if you disrupt both the neuroligin gene and the neurexin gene, the worms show even less social feeding behavior than if you disrupted just one of them (Figure 1C).
Next, Mara and her group wanted to take a closer look at where exactly the neurexin and neuroligin genes were acting to support social feeding behavior. Based on previous work, they knew that a few select neurons are particularly important in social feeding. Interestingly, Mara found that these neurons express the neurexin and neuroligin genes and that expressing neurexin in just two of these neurons restored social feeding behavior. Further, Mara wanted to know how neuronal communication was affected in worms that lacked these genes. She found that in social worms without neurexin there were fewer points of connections, known as synaptic puncta. Additionally, these neurons release a chemical called glutamate which is known to be very important for neurons to signal to one another. Mara and her group found that more glutamate was released from one particular social feeding neuron in the social control group and less glutamate was released from that same neuron in the solitary control group. Mara’s work suggests that increased neural communication, be that number of synaptic puncta or amount of glutamate, was associated with increased social feeding behavior. Therefore, this work provides insight into how genes that regulate the connection between neurons impact social behavior.
Taken together, Mara’s results point to multiple underlying factors that have a direct impact on social feeding behavior. In particular, she identified neurexin, neuroligin, and glutamate as key biological components that are important for this behavior. And importantly, some of these same biological factors have been implicated in human neurodevelopmental conditions, such as autism. Future experiments will build off Mara’s work to get a clearer picture of how social behavior emerges from these biological factors and even perhaps lead the way to the development of therapeutics to help improve people's lives.
About the brief writer: Jafar Bhatti
Jafar Bhatti is a PhD Candidate in the lab of Dr. Long Ding / Dr. Josh Gold. He is broadly interested in brain systems involved in sensory decision-making.
Interested In learning more? Check out the full paper here!
Brain cells communicate to help us find food
or technically,
Distinct neurexin isoforms cooperate to initiate and maintain foraging activity
[See original abstract on Pubmed]
Brandon Bastien was the lead author on this study. Brandon graduated from Swarthmore College with a degree in neuroscience with high honors. He then obtained his PhD in Neuroscience from the University of Pennsylvania in the Hart Lab and has continued his work as a postdoctoral researcher. Since joining the Hart lab, his focus has been on defining the circuitry underling how C. elegans respond to food deprivation stress and how certain synaptic proteins, some of which have orthologs in humans (like neurexins and casprs), and neuromodulators may distinctly act within this behavior-generating circuit. Outside of lab, he enjoys TV shows, football, Eurovision, and traveling.
or technically,
Distinct neurexin isoforms cooperate to initiate and maintain foraging activity
Figure 1: Neurons may communicate through chemical synapses. The adhesion molecules and molecules dictates the synaptic communication.
[See Original Abstract on Pubmed]
Authors of the study: Brandon L. Bastien, Mara H. Cowen, and Michael P. Hart
The World Jigsaw Puzzle Championship is an annual event where individuals, pairs, and teams compete to solve puzzles the fastest. In the final team round of the Puzzle Championship, groups race each other to solve two 1000 piece puzzles the fastest.
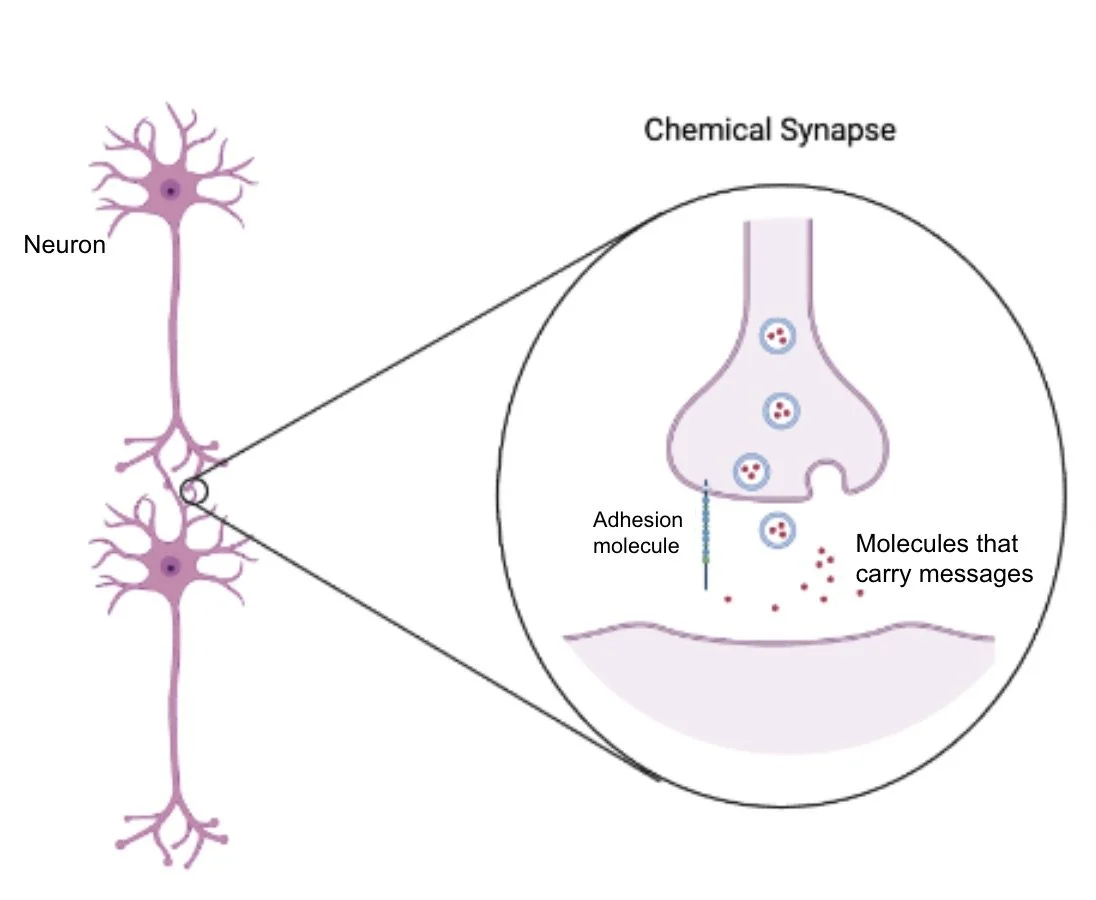
Neuroscientists around the world are also puzzlers, of a slightly different variety. The puzzles they aim to piece together are the human brain and other nervous systems/brains. The human brain contains billions of pieces each fitting together to reveal an image of who we are. In the brain, each piece is a cell, a basic unit. Just like border pieces and ribbon pieces are different types of puzzle pieces, the brain has different types of pieces/cells as well. One type of brain cell is called neurons. Neurons communicate with each other by forming connections through the small gaps called synapses. The main type of synapse is chemical synapses. In the chemical synapses, the brain uses molecules which are chemicals that are transferred from one neuron to another passing along messages. One neuron releases the chemicals which travel across a small gap before binding to the receptive neuron to transmit signal/information. Chemical synapses may be modified by structural properties, including how close together the neurons are at the synapse, how large the synapse is, and more. Synaptic structure is held together using adhesion molecules that form bridge-like structures that facilitate and modify communication at the synapse (Figure 1). The synaptic structures may modify how the chemical molecules messengers communicate with the receptive neuron. The string of specific neurons fitting in with each other makes neural circuits. The circuits have a function of regulating behaviors such as walking, eating, or sleeping. When neuroscientists are able to understand how our neuron pieces fit together through synapses to regulate behavior, for even a small part of the brain, it is also a win with the price of improving human lives.
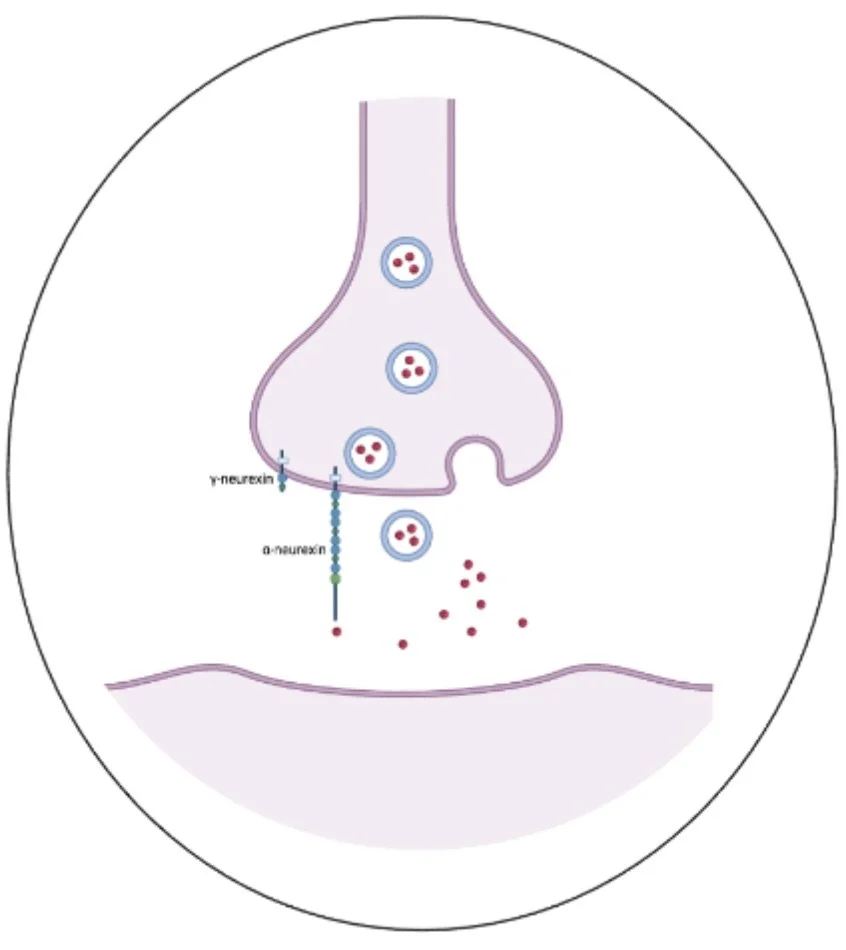
Figure 2: γ version and α isoforms of neurexin in a synaptic structure
Neurexin, a synaptic structure, forms part of the bridge between neurons to enable communication. There are different versions of neurexin. Alterations to neurexins affect many behaviors associated with neurodiversity, such as autism spectrum and schizophrenia. It is important to understand how neurons communicate through synapses to mediate circuits that regulate behaviors.
Brandon Bastien, a NGG graduate, focuses on understanding how brain pieces fit together through the synapse to regulate behavior, specifically foraging behavior. Foraging behavior, the search for food is an essential behavior that is conserved in all animals. Since the human brain has billions of pieces, Brandon uses a smaller animal, a small worm C. elegans that has just 302 puzzle pieces (compared to the human brain, think of this as a puzzle for a child). C. elegans also have conserved genetic or hereditary information, synaptic structure, synaptic molecules, and foraging behaviors. In the worm the piece that is known to drive foraging behavior is the pair of neurons named RIC (one on left and one on right). It was known that the RIC neurons use chemical synaptic messenger octopamine for foraging behavior. It was unknown if the synaptic cell adhesion structure neurexin is important for foraging behavior or the connections made to or by RIC neurons
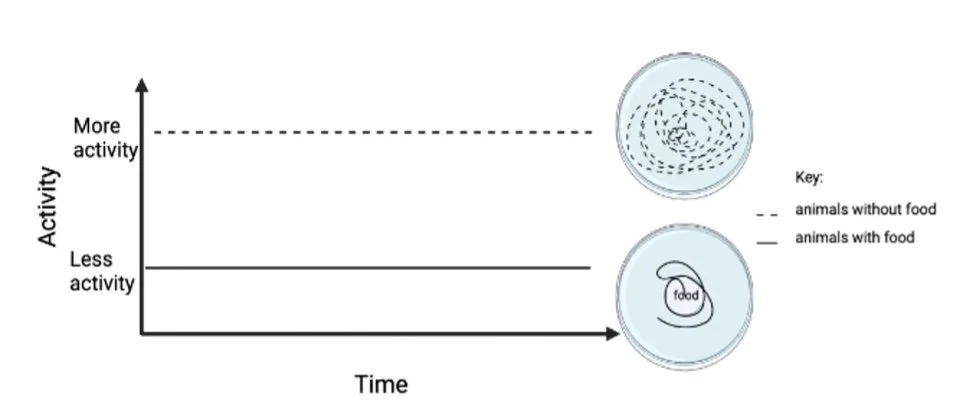
Brandon asked how the synaptic protein neurexin affects the foraging behavior of C. elegans. Two versions of neurexin in the human and the worm are called γ version and α isoforms (Figure 2). The two different versions are both located in the synapse and have shared parts, but are different sizes (one longer and one much shorter). He used genetic manipulations by deleting the different versions of neurexin hereditary information to test if neurexin modifies foraging behaviors. Foraging behavior, the search for food, is quantified by the high activity level of the animal. The worms were placed in environments without food and were videotapes with a microscope over the course of eight hours to evaluate their activity. Higher activity indicates food search and a lower activity indicates less food search. Similarly to humans, we will move source food when we are hungry. The movement of the worms off food was compared to animals that were on food. Animals without food will have a higher movement activity to look for food and the animal with food moved less since they did not need to find food (Figure 3 ).
Figure 3: animals that are food deprived have more activity than animals with food.
Brandon identified the roles for different neurexin versions in foraging behaviors by asking how the food searching changed when he deleted them. When the short γ version of neurexin was deleted, in the absence of food, the animals had a temporal effect. Within the first hour, animals that were missing the short γ version of neurexin and were food deprived had comparable movement when compared to animals that were fed. However, from hours 2-8, animals that were missing the short γ version of neurexin and were food deprived increased their movement significantly when compared to feed animals. This indicates that the γ version of neurexin is important for starting the food search behavior, but it is less important for maintaining the later hours of food search. When the α version of neurexin was deleted, in the absence of food the worm moved more than the worms that had food in the first hour in an attempt to find food, but then decreased their movement in the later hours for the entire eight hours. This implies that the α version of neurexin is important for maintaining food search behavior, but is less important for starting the food search behavior. This is the first time that the different versions of neurexin were found to both impact foraging behaviors. The different versions of neurexins have different functions in the food search behavior with γ version of neurexin being important for starting food search and the α version of neurexin being important to maintain continued food search (Figure 4). The different forms of neurexin cooperate through different functions and cooperate to start and continue this foraging behavior.
Figure 4: The γ version of neurexin is important for starting the food search behavior, while the α version of neurexin is important for maintaining food search behavior.
The next question Brandon asked was how were the different forms of the synaptic structure neurexin affecting different phases of the foraging behavior interact with the chemical synaptic signaling molecule octopamine. To test this question, Brandon used animals that were missing octopamine. In animals that were missing octopamine in the absence of food, the worms had less activity in the first two hours then had increased activity in the remaining eight hours when compared to animals on food. This indicates that the synaptic molecule octopamine is also important for starting food search behaviors, but is not required for maintaining food search behaviors. Therefore, both the γ version of neurexin and octopamine signaling are both essential for starting foraging behavior. Additionally, neurexin in neurons is needed for octopamine function. May want to mention that when branded deleted the alpha isoform, there were fewer RIC synapses - referring back to intro on chemical synapse structure?
Brandon has shown that RIC neurons have synapses that have synaptic structure neurexin and synaptic molecule octopamine that functions in regulating foraging behavior. In addition, the paper identified the different and collaborative roles the different forms of neurexin have in foraging behaviors with the γ version starting foraging behaviors and the α version of neurexin maintaining the foraging behavior in the absence of food. Lastly, Brandon showed that the synaptic structure neurexin is required for the synaptic molecule octopamine to function showing how team work in synapses regulates behavior. This work provides insights into how neurons form communication through synapses to alter circuits that regulate behavior. Neuroscientists have more work to do in understanding how the different puzzle pieces of our brain fits together.
About the brief writer: Sophia M. Villiere
Sophia is a PhD Candidate in Michael P. Hart’s lab in the Neuroscience Graduate Group at the University of Pennsylvania, studying how autism associated gene mutations affect neuron morphology and behavior.
Interested in learning more about foraging behavior? Click on Brandon’s full paper here
The inner workings of a rare childhood disease
or technically,
Altered lipid homeostasis is associated with cerebellar neurodegeneration in SNX14 deficiency
[See original abstract on Pubmed]
Vanessa Sanchez was the lead author on this study. As a scientist, Vanessa is passionate about understanding the cell biology of neurons, especially in the context of pediatric neurodevelopmental – degenerative disorders. Outside of lab, you can find Vanessa tending to her garden, trying new recipes, rock climbing, or hanging out with her cat, Jiji!
or technically,
Altered lipid homeostasis is associated with cerebellar neurodegeneration in SNX14 deficiency
[See Original Abstract on Pubmed]
Authors of the study: Yijing Zhou, Vanessa B Sanchez, Peining Xu, Thomas Roule, Marco Flores-Mendez, Brianna Ciesielski, Donna Yoo, Hiab Teshome, Teresa Jimenez, Shibo Liu, Mike Henne, Tim O'Brien, Ye He, Clementina Mesaros, Naiara Akizu
Neurons are special cells in our bodies that communicate with one another to help us do everyday things like eat, think and walk. Amazingly, for most healthy people, the neurons that we are born with will last our lifetimes and support us as we navigate the world. However, in some rare and unfortunate diseases, neurons die prematurely. These kinds of diseases are called neurodegenerative diseases. There are many different types of neurodegenerative disease, each targeting different groups of neurons and resulting in different symptoms. In 2014, a new and extremely rare neurodegenerative disease was discovered called SCAR20. SCAR20 was found to negatively affect newborn children by causing intellectual disability and impairing motor functions, like the ability to walk. Researchers were quickly able to identify the culprit of the disease: the total lack of a protein called SNX14. Since little is known about SNX14 and how its absence causes SCAR20, Vanessa Sanchez, a current NGG student, and her collaborators designed a study to learn more about the nature of this disease, with the hope that one day there might be a cure or treatment.
Figure 1. Key takeaways from Vanessa and colleagues’ experiments investigating the underlying causes of the SCAR20 disease.
To begin their investigation, Vanessa and her collaborators used genetic tools to remove the SNX14 protein from mice. Genetically modified mice are immensely useful in neuroscience research as they allow scientists to study the underlying causes of disease in detail. In this case, since the researchers removed a protein, the genetically modified mice are referred to as a knockout mouse model. After they generated their new knockout mice, Vanessa and her colleagues tested these mice to make sure that they had all of the symptoms that the children experienced. This was an important step in their study because they wanted to be sure that any discoveries that they make using the knockout mouse model are directly relevant for human patients. Vanessa and her colleagues compared the knockout mice to normal healthy mice and found a few convincing results (Figure 1, Healthy mouse vs. Knockout mouse). First, they found that knockout mice had a complete lack of SNX14 in their brains - the direct cause of SCAR20 in humans. Next, they found that knockout mice were smaller in size and had a structurally abnormal face - two known symptoms of SCAR20 in humans. Finally, they found that the knockout mice had worse social memory and motor ability compared to healthy mice - again, a clear-cut sign of SCAR20 in humans. Given these results, Vanessa and her colleagues were convinced that they had developed a good mouse model of the SCAR20 disease and were now able to investigate how the disease develops.
In order to gain insight into the underlying causes of the disease, Vanessa and her colleagues needed to narrow down their focus to a single brain area. In human patients, SCAR20 seems to preferentially kill neurons in a brain area known as the cerebellum. This brain area is typically thought to be involved in motor control and coordination, which might explain why SCAR20 patients have severe motor disability. Vanessa and her colleagues discovered that, just as in human SCAR20, the knockout mouse model also showed a preferential negative effect on the cerebellum of the mice. She found that both the number of neurons and the overall size of the cerebellum were reduced in the knockout mice compared to healthy mice, once again validating the model for the study of SCAR20 and identifying a key brain area to narrow in on.
At this point, Vanessa and her colleagues have all the tools they need to study the inner workings of the disease. They performed a very important experiment where they extracted neurons in the cerebellum of knockout mice before they were killed by the disease, and looked for differences compared to the neurons in the cerebellum of healthy mice (Figure 1, Healthy neuron vs. Knockout neuron). By testing various cell properties, they discovered that there was one key cell property that was disrupted in the neurons of knockout mice compared to the neurons of healthy mice. This key cell property is called lipid homeostasis, which is important for regulating lipids, the building blocks of fat, inside the cell. Despite what you may expect, fats play an essential role in cell biology. Disrupting the total amount of fats inside of the cell can be toxic, resulting in cell death. In fact, Vanessa and her colleagues discovered that knockout neurons had trouble removing fats from the cell, resulting in a build-up. They went on to show that this disruption in lipid homeostasis is most likely the root cause of neuron death in SCAR20, which underlies the known symptoms of the disease.
This important research by Vanessa and her colleagues sheds light on the inner workings of a new disease that severely impacts the well-being of newborn children. Although there is still much to learn about the nature of this disease, such as how it affects neurons in other brain areas, the findings from Vanessa’s experiments offer a strong foundation for the possible development of treatments for this debilitating disease. Finally, research like Vanessa’s is invaluable as it contributes to our basic understanding of how neurons work and what causes them to die prematurely - knowledge that is fundamental for all neurodegenerative diseases.
About the brief writer: Jafar Bhatti
Jafar Bhatti is a PhD Candidate in the lab of Dr. Long Ding / Dr. Josh Gold. He is broadly interested in brain systems involved in sensory decision-making.
Interested in reading more? See the full paper here!
Why some people wake up under anesthesia and others don’t (Hint: It’s your Hormones)
or technically,
Hormonal basis of sex differences in anesthetic sensitivity
[See original abstract on Pubmed]
Dr. Andrzej (Andi) Wasilczuk was the lead author on this study. A recent graduate from Penn Bioengineering, Andi is captivated by the brain’s intricate ability to shift between states of consciousness. His research uses general anesthetics to uncover the neuronal circuits responsible for these shifts, aiming to understand how the brain sustains or disrupts consciousness. By identifying these critical networks, Andi is paving the way for personalized anesthesia and offering new insights into arousal state transitions.
or technically,
Hormonal basis of sex differences in anesthetic sensitivity
[See Original Abstract on Pubmed]
Authors of the study: Andrzej Z Wasilczuk, Cole Rinehart, Adeeti Aggarwal, Martha E Stone, George A Mashour, Michael S Avidan, Max B Kelz, Alex Proekt, ReCCognition Study Group
Waking up during surgery sounds like a nightmare, and did you know that females might be at higher risk for this than males? Through medication, general anesthesia makes a patient unconscious, which allows doctors to perform surgical procedures without the patient’s awareness or discomfort. General anesthesia puts the patient in a sleep-like state, and doing this is an involved process. Anesthesiologists must be highly trained to determine the best course of treatment. When creating a safe treatment plan, anesthesiologists take into account many factors, such as the patient’s body weight or pre-existing conditions. The sex of the patient, however, hasn’t been historically considered as an equally important factor in delivering a safe course of anesthesia.
Previous research about the link between sex and response to anesthesia was ambiguous and conflicting. Some early clinical trials suggested that females were more likely to wake up under anesthesia, while others found no significant difference between males and females. These clinical trials, however, had diverse patient populations and non-standardized anesthetic protocols, which would make it hard to directly compare anesthetic conditions between patients. Nevertheless, more recently, an analysis done on many of these studies has provided clear cut evidence that females are more resistant to the anesthetic state (Braithwaite et al., 2023). The question of how this sex difference arose, however, remained unanswered.
Dr. Andi Wasilczuk, a former Penn Bioengineering PhD student, and his team wanted to understand why females and males responded differently to anesthesia. To do this, they decided to focus on the hypothalamus, a structure in the brain heavily involved in both sleep-wake and anesthetic induced unconsciousness. The hypothalamus is regulated by hormones, which are the body’s chemical messengers—and the researchers knew that the levels of hormones typically differ between males and females. For example, males typically have a much higher level of the hormone testosterone, whereas females typically have higher levels of the hormone estrogen.
With these differences in mind, Dr. Wasilczuk wanted to know: Could hormonal differences across sexes alter the effectiveness of general anesthesia? He framed "effectiveness of general anesthesia" by using the idea of “anesthetic sensitivity.” Individuals who are more sensitive to anesthesia need less of the drug to fall and stay unconscious, and wake up smoothly after surgery. On the contrary, individuals with less anesthetic sensitivity, or have anesthetic resistance, require more anesthetic to fall and stay asleep, and wake up sooner once the anesthetic is removed.
Recognizing this gap in the research, Dr. Wasilczuk’s research group sought to test the influence of sex and sex hormones on anesthetic sensitivity in mice. First, the researchers compared the dosage of anesthetic required for the mice to be initially anesthetized (induction), and to wake up from anesthesia (emergence). They found that, across all four anesthetics the group tested, female mice required a much higher dose on induction, and were more likely to emerge at higher doses than males. Next, the researchers compared the time, given the same dosage, for female versus male mice to be induced and emerge from anesthesia. Female mice took significantly longer to be induced than males, and also were much quicker to emerge. These experiments indicated that female mice were indeed more resistant to anesthesia, compared to male mice.
Yet, the reason for these results remained unclear: Were these effects due to sex hormone differences? To find out, the researchers changed the mice's hormone levels by surgical removal of the testicles (castration) in male mice or ovaries (oophorectomy) in female mice post puberty. They repeated the experiments, this time using castrated males and oophorectomy females, then compared these mice to the untreated males and females tested before.
The results were striking. In both experiments, castrated males and oophorectomized females showed a similar resistance to anesthesia as untreated females. Oophorectomy did not change a female mouse’s anesthetic sensitivity. Castration, however, produced a female-like anesthetic sensitivity in males. Eliminating male sex hormones, therefore, seemed to remove the sex differences in response to anesthesia!
The researchers also directly measured the effect of testosterone. Under a steady dose of anesthetic, untreated males and castrated males were injected with testosterone, and continually tested for responsiveness using the righting reflex. Testosterone administration increased anesthetic sensitivity for both groups of mice in a dose-dependent manner. This finding could explain why males, who typically have higher testosterone, are more sensitive to general anesthetics, and therefore are at lower risk of waking up under anesthesia than females.
Intrigued, the researchers wondered: Can these sex differences be seen in brain activity? The conventional measure of anesthetic depth (how unconscious someone is in response to anesthesia) during surgery is the Electroencephalogram (EEG). EEG measures electrical brain activity through electrodes attached to the scalp. The researchers found that sex differences were not reflected in the EEG of the mice they tested. Similar conclusions were made when re-analyzing human data from another study. In this study, female volunteers displayed resistance to general anesthesia based on assessments of behavior and cognitive function, but not based on information gathered from the EEG.
Looking at the activity of individual neurons, however, clearly revealed sex differences. They looked for elevated levels of the protein c-Fos, an indicator of neuronal activity, throughout the whole brain. Compared to anesthetized male mice, anesthetized female mice had fewer neurons expressing c-Fos in sleep-promoting hypothalamic cells. In other words, anesthesia activates fewer sleep-promoting circuits in females than males, correlating with females’ greater resistance to anesthetics.
Compared to untreated male mice, castrated male mice also had reduced c-Fos expression in similar hypothalamic structures. Fewer sleep-promoting circuits were activated in castrated males (which displayed a similar aesthetic sensitivity to females) than untreated males. Thus, sex-dependent activity patterns, seen in hypothalamic structures, reflected anesthetic sensitivity trends!
Dr. Wasilczuk’s groundbreaking paper reveals why researching sex-dependence is incredibly important: females may need different anesthetic management than males due to their higher resistance to anesthesia. After years of standard general anesthesia administration to millions of patients, and using EEGs to measure anesthetic depth, Dr. Wasilczuk’s findings have huge clinical implications supporting personalized anesthetic care.
About the brief writer: Sydney Liu
Sydney is a guest writer for Brains in Briefs! She is a Penn undergraduate in Dr. Shinjae Chung’s lab researching what makes us sleep and the brain transitions between sleep states. She is a Junior majoring in neuroscience, and is interested in teaching. In her free time, she likes to draw!
Citations:
E. Braithwaite et al., Impact of female sex on anaesthetic awareness, depth, and emergence: A systematic review and meta-analysis. Br. J. Anaesth. 131, 510–522 (2023).
Interested in learning more about how anesthetic sensitivity is different in males and females? Check out Andi’s paper here!
Finding the patterns of white matter growth that support children’s cognitive development
or technically,
Development of white matter fiber covariance networks supports executive function in youth
[See original abstract on Pubmed]
Joëlle Bagautdinova was the lead author on this study. Joëlle is broadly interested in brain development and how this may go awry in psychiatric disorders. For her PhD in Dr. Ted Satterthwaite’s lab, Joëlle is using neuroimaging to study the mechanisms underlying brain development, cognition and psychiatric disorders. She is particularly interested in understanding the potential role of sleep as a risk factor in the emergence of mental illness.
or technically,
Development of white matter fiber covariance networks supports executive function in youth
[See Original Abstract on Pubmed]
Authors of the study: Joëlle Bagautdinova, Josiane Bourque, Valerie J. Sydnor, Matthew Cieslak,Aaron F. Alexander-Bloch, Maxwell A. Bertolero, Philip A. Cook, Raquel E. Gur, Ruben C. Gur, Fengling Hu, Bart Larsen, Tyler M. Moore, Hamsanandini Radhakrishnan, David R. Roalf, Russel T. Shinohara, Tinashe M. Tapera, Chenying Zhao, Aristeidis Sotiras, Christos Davatzikos, and Theodore D. Satterthwaite
Recently, many neuroscientists have been trying to uncover the developmental “blueprint” of the brain’s gray matter, or the specific ways in which brain regions grow and change over the course of adolescence. However, less attention has been paid to the brain’s white matter, which is the insulated, wire-like “tracts” that connect one brain region to another. NGG student Joëlle Bagautdinova and her colleagues in the Satterthwaite lab filled this gap by investigating white matter’s structural development in MRI scans from almost 1000 people ages 8 to 22 years.
While it famously does NOT imply causation, correlation can show parts of the brain have similar structures and, therefore, might be following the same developmental blueprint. So, Joëlle and her colleagues decided to cluster every point along the brain’s white matter tracts (Figure 1) into groups with similar structures (Figure 2). Specifically, they grouped points with similar fiber density, or how many “wires” are packed together to make the tract, and cross-section, or how thick the tract is (Figure 1); they refer to the combination of these measurements as “FDC”. She also tested to see how each group’s FDC values changed across adolescence.
Figure 1. White matter tracts can be measured by their density and cross-section.
Usually, researchers assume that all points along a tract will develop similarly; however, because Joëlle determined her groups based on how similar the points are, different points along the same tract could be put into different groups, while points from more than one tract could be lumped together. This allowed her to uncover brand new relationships between different white matter tracts and unique subsections that develop differently than the white matter tract. For instance, she found that FDC in the lower part of the corticospinal tract, which connects the brain and spinal cord, was different than the FDC in the upper corticospinal tract, and each portion had its own unique growth trajectory. All in all, the researchers found 14 different groups of similarly-structured white matter regions, 12 of which showed significant structural changes across this period of adolescent development.
The age at which each white matter group developed most also seems to follow a pattern. Specifically, they found that the white matter in the lower back area of the brain matures earlier in adolescence while the white matter in the upper front area of the brain doesn’t mature until a bit later. These early-maturing white matter tracts tend to connect parts of the brain that do what scientists call “lower-order functions” like vision processing, basic movement, and emotions – all things that children can do pretty well. Meanwhile, the later-maturing white matter tracts tend to connect brain regions that do “higher order” functions like complex reasoning. Overall, the fact that white matter maturation seems to progress “basic” to “complex” tracts suggests that white matter may play a big role in the brain’s development across adolescence.
Figure 2. Points of white matter can be grouped by how similar their FDC (fiber density and cross-section) values are.
Finally, Joëlle and her colleagues wanted to see if these white matter structures helped kids’ executive function, which is one of these “higher order” cognitive functions that includes planning, organizing, and impulse control. They found that if you remove the effects of age, kids with better executive function tend to have higher FDC in all but one white matter group. This means that white matter tracts that are thicker and/or more tightly packed do a better job of sending signals between brain regions, especially those in the front of the brain that are responsible for cognition, and that this enhanced signaling may allow children to have stronger executive functions.
By using new, cutting-edge analyses, Joëlle and her collaborators were able to: uncover brand-new, biologically-based relationships between white matter areas; chart how these areas develop over adolescence; and show which white matter structures seem to help with cognitive function. All in all, this work fills in important gaps in our understanding how the brains we’re born with mature into the brains of capable, full-grown adults.
About the brief writer: Margaret Gardner
Margaret is a PhD student in the Brain-Gene-Development Lab working with Dr. Aaron Alexander-Bloch. She is interested in studying how different biological and demographic factors influence people’s brain development and their risk for mental illnesses.
Want to learn more about this exciting research? Check out Joëlle’s paper here!
A bark or a grunt? A look at what makes animal calls sound different
or technically,
Time as a supervisor: temporal regularity and auditory object learning
[See original abstract on Pubmed]
Ron DiTullio was the lead author on this study. He is a computational neuroscientist who studies how the brain transforms information about the outside world into efficient and useful neural representations. His current work focus on spatial navigation and memory, audition, and social cognition with future work planned to focus on vision.
or technically,
Time as a supervisor: temporal regularity and auditory object learning
[See Original Abstract on Pubmed]
Authors of the study: Ronald W. DiTullio, Chetan Parthiban, Eugenio Piasini, Pratik Chaudhari, Vijay Balasubramanian, Yale E. Cohen
As you walk through the parking lot it probably doesn’t take much effort to recognize the laughter of your friends behind you, the gentle hum of a car engine rolling down the neighboring aisle, or the clatter of wheels rolling on someone’s shopping cart. Each one of these sounds is what neuroscientists call an auditory object. While we can often effortlessly recognize auditory objects, determining exactly how we do so is difficult. It might be obvious to us that a particular sound is a car engine or a laugh, but we would be hard-pressed to name exactly what features of the sound distinguish one from another. That’s why recent NGG graduate Dr. Ronald DiTullio and a team of researchers at the University of Pennsylvania set out to figure out what makes auditory objects sound different.
Identifying the features that define auditory objects has at least two important applications in neuroscience and beyond. First, it might give experimenters clues as to how the brain learns to differentiate sounds. For example, once researchers have identified features that separate human voices from the sound of kitchen appliances, they can look at brain activity and ask whether the brain learns the same features. Second, being able to measure and quantify useful features of sounds allows scientists to build artificial systems that can distinguish them as well. For example, your Google Home or Amazon Alexa system might benefit from looking for features that will help it learn to distinguish your voice from a tea kettle boiling in the background.
DiTullio and his team aimed to find the features that differentiate auditory objects by studying the different types of vocalizations that rhesus macaque monkeys make. They chose to study macaque vocalizations because they have structure, just like human speech, and vocalizations can be grouped into a small number of types. In this case, analyzing the differences between macaque vocalizations is easier than looking at something as complicated as spoken language. “There is a general pattern in nature,” DiTullio explained. “We know that macaque vocalizations should share similar structure with human vocalizations.” Because sounds in nature share similar features, the team hopes that what they learn about the differences between macaque vocalizations will apply to other types of sounds.
The research team started with a key observation: the sounds that make up auditory objects change slowly and smoothly over time. This is a property called temporal regularity. We can understand the concept of temporal regularity by thinking about playing notes on the piano. When you play and hold a note, the note fades away slowly and the components of the sound are largely the same over time. This example has high temporal regularity. However, if you randomly play and release a note, you quickly switch between silence and the note and the components of the sound change a lot from moment to moment. Thus, this example has low temporal regularity. That is one way DiTullio and his team think we can distinguish one note from another. Temporal regularity is a known feature of many auditory objects, including macaque vocalizations. “The underlying idea is to find a pattern we think exists in natural sounds and that the brain could get useful information from,” said DiTullio. “Our main motivation was to ask, ‘is temporal regularity that pattern?’”.
To test their idea, the team first demonstrated that temporal regularities can be used to distinguish auditory objects. To do this, they took recordings of four different types of macaque vocalizations (coo, harmonic arch, shrill bark, and grunt) and three different types of noise and used statistical methods to quantify different features of each audio clip. One of these statistical methods, called slow feature analysis, looked for temporal regularities in the audio clips, whereas the other methods relied on different types of features. The group found that the temporal regularities identified by slow feature analysis did a better job of distinguishing auditory objects than the features identified by the other statistical methods. This showed that temporal regularities are in fact an important feature of macaque vocalizations that can be used to differentiate them.
The team next applied their idea to another challenge for our auditory systems: the ability to recognize auditory objects in the presence of noise. For example, most people will have no problem distinguishing the statement “I have plans” from “I have plants” in a quiet room, but that becomes much more difficult when having a conversation at a crowded cocktail party. To test whether temporal regularities might help solve this problem, the team applied the same statistical methods discussed previously to audio clips of the same types of macaque vocalizations, but with noisy backgrounds. They found that the temporal regularities identified by the slow feature analysis did a better job than any other features at distinguishing macaque vocalizations with noisy backgrounds. The team showed that temporal regularities are a useful way the brain might solve the problem of identifying sounds in the noisy environments we encounter in our everyday life.
Taken together, these experiments identify an important feature of auditory objects that is useful for distinguishing them: temporal regularity. This opens the door to lots of exciting future work. One open question is whether the brain uses the same features when learning the differences between auditory objects. “We hope that more people will look for these kinds of signals in the brain,” said DiTullio. “People know that temporal regularities are an important part of audition, but they don’t necessarily look for how... the brain might learn these [features].” Another interesting direction for future work would be to investigate different types of sounds, like spoken language. DiTullio’s team has already done follow-up work applying these ideas to bird and human vocalizations. It seems like this is only the start of what temporal regularities may be able to teach us about what makes the sounds we encounter every day sound so different.
About the brief writer: Catrina Hacker is a PhD candidate working in Dr. Nicole Rust’s Lab. She is broadly interested in the neural correlates of cognitive processes and is currently studying how we remember what we see.
Learn more about how the team determined what distinguishes different types of sounds in the original paper.
Neurons in the brainstem promote REM sleep and trigger brainwaves that might cause dreaming
or technically,
A medullary hub for controlling REM sleep and pontine waves
[See original abstract on Pubmed]
Dr. Amanda (Mandy) Schott was the lead author on this study. As a researcher, Mandy is most interested in defining neural circuits, and how specific populations of cells communicate to generate essential human behaviors such as sleep.
or technically,
A medullary hub for controlling REM sleep and pontine waves
[See Original Abstract on Pubmed]
Authors of the study: Amanda Schott, Justin Baik, Shinjae Chung & Franz Weber
Rapid eye movement (REM) sleep is the sleep state that most people associate with dreaming, however REM sleep has many other essential functions. While REM makes up only about 20-25% of our nightly sleep, it is vitally important for memory, emotional processing, and other functions we have yet to understand. This is true not just for humans, but all mammals and maybe even birds and reptiles! To facilitate all these functions of REM, the brain is highly active during this sleep state. In fact, during REM sleep, brain signals look more similar to wake than non-REM sleep. Because of this, REM sleep is sometimes called paradoxical sleep because paradoxically, the brain is so active during rest.
Surprisingly, we still know very little about how the brain switches from low-activity non-REM sleep to high-activity REM sleep. Moreover, during REM sleep there are sometimes sporadic brain waves that seem to be important for normal brain function but whose precise role is still not totally clear. P-waves are one such waveform that is caused by lots of synchronous neuronal activity in the back of the brain, in a brainstem region called the pons. From the pons, P-waves travel forwards in the brain to brain regions important for forming and storing memories, and also areas involved in visual processing. These P-waves are interesting because they occur only during REM sleep, and are proposed to be involved in dreaming and the memory functions of REM sleep. A paper by recent NGG graduate Dr. Amanda Schott investigated two major unknowns in REM sleep research: 1) What neurons and brain regions are involved in generating REM sleep, and 2) What neurons and brain regions are involved in generating P-waves. Is it possible that one set of neurons could do both?
While we know of several brain regions in the brainstem that regulate REM sleep, most of them consist of inhibitory neurons, meaning they “turn off’ other brain regions to promote REM sleep. Dr. Schott, however, found a highly unusual group of excitatory neurons in part of the brainstem called the dorsal medial medulla (dmM). These excitatory neurons can “turn-on” other neurons they make connections with. These dmM excitatory neurons were only active during REM sleep, suggesting they may be involved in promoting REMs sleep. In addition, dmM neurons project their axons and send signals to the part of the pons that is known to generate p-waves. In fact, the dmM neurons were active at the same time the p-waves occurred suggesting that the dmM excitatory neurons could be involved in the generation of p-waves too! Dr. Schott next wanted to directly manipulate the activity of these neurons to see if they could cause transitions to REM sleep or cause generate p-waves.
Using a modern neuroscience technique called optogenetics, Dr. Schott was able to cause the neurons in the dmM to fire when a laser light was shined over them through an optic fiber. She simultaneously determined if the mouse was awake, asleep, or in REM sleep by measuring the mouse’s brain waves using electroencephalography, or EEG. She found that stimulating these neurons caused the mouse to enter REM sleep, and also increased the length of REM sleep episodes. Shining the laser light also caused a p-wave to be generated when the light was shined about 60-100% of the time when the mouse was sleeping. Experimentally reducing the activity of the dmM neurons also decreased the amount of REM sleep, as well as the amount of p-waves. Dr. Schott interpreted these findings as evidence that dmM excitatory neurons are critical for normal amounts of REM sleep to occur, and for triggering p-waves.
Overall, Dr. Shott’s work adds an important piece to the puzzle to our understanding of which brain regions can promote REM sleep. Her findings are an important first step in understanding which neurons generate p-waves which is ultimately necessary to understand p-wave function. This work will provide a foundation on which others (including the author of this piece!) can study the role of p-waves in REM sleep, and move closer to finally understanding how and why we dream.
About the brief writer: Emily Pickup
Emily is a 4th year PhD candidate in Dr. Franz Weber’s lab. She is interested in the biological functions of sleep. Specifically, she is interested in understanding the function of REM-specific p-waves. The large pontine waveform implicated in memory consolidation discussed in the brief above.
Interested in learning more about REM sleep and p-waves? See the original paper here.
Does zip code contribute to racial health disparities in aging?
or technically,
Contributions of neighborhood social environment and air pollution exposure to Black-White disparities in epigenetic aging
[See original abstract on Pubmed]
Isabel Yannatos was the lead author on this study. They studied racial disparities in aging, examining how the external environment is internalized to influence health outcomes among Black and White Americans. Currently, they are pursuing a career in health policy to move towards health equity.
or technically,
Contributions of neighborhood social environment and air pollution exposure to Black-White disparities in epigenetic aging
[See Original Abstract on Pubmed]
Isabel Yannatos, Shana Stites, Rebecca Brown, Corey McMillan
What’s the first thing that comes to mind when you think of health? A doctor’s office? A waiting room? A pharmacy? Whatever image you conjured, your house and your surrounding neighborhood probably didn’t make the cut, but the environments in which we live, work, and age are actually huge determinants of our health.
These “environments” encompass not just the environment in a traditional sense (exposure to severe weather or air pollution), but also the social (violence/crime, sense of community, and wealth) and physical (walkability or access to green spaces) elements of your neighborhood. All of these factors play a critical role in how healthy you are and how well you age [1]. However, all environments aren’t created equally, and long-standing racism and exclusionary policies directly influence how these important health determinants, like access to healthy food or exposure to pollutants, are distributed.
For instance, it is well established that Black Americans have fewer socioeconomic resources and higher exposure to unhealthy conditions in their neighborhoods [2]. In turn, these environments create and cement a number of racial health inequities such as increased prevalence of age-related diseases, like Alzheimer’s [1-3]. This disparity in aging is not due to genetic differences, rather to differences in socioeconomic risk factors, inadequate healthcare access (e.g., decreased management of conditions like high blood pressure and diabetes), and the cumulative impact of social stress and political marginalization [3].
Motivated to further understand how neighborhood discrimination influences health and aging, former Neuroscience Graduate Group (NGG) student Dr. Isabel Yannatos and colleagues from the Bioinformatics in Neurodegenerative Disease lab leveraged a large (2,960 participants!) dataset collected by the National Institute on Aging. This Health and Retirement Study consisted of phone or in-person surveys collected from a representative population of Americans 50 years and older.
In addition to filling out these surveys, which asked individuals about their race/ethnicity, age, and social and physical environment, a subset of participants gave a blood sample for measurement of DNA methylation. Methylation is a process by which the body adds little chemical tags to specific parts of DNA molecules. Typically, these methylation tags prevent part of the DNA molecule from being used by the body. This to say, methylation can change the activity of the DNA molecule without changing the DNA sequence. Accumulating DNA methylation tags is a normal part of aging. It’s one way our bodies -- and our DNA -- change as we get older. In fact, scientists can use the pattern of methylation present on a person’s DNA to calculate their biological age. The difference between this biological age (e.g., DNA methylation-based age) and chronological age (e.g., the number of years someone has been alive) is one way to measure the pace at which someone is aging [4]. If biological age comes out to be higher than chronological age, it’s a sign that the body is aging more quickly than expected.
Isabel and colleagues used measures of DNA methylation to calculate a biological age for each participant. They found that Black participants appeared biologically older than White participants of the same chronological age, suggesting that Black Americans are aging more quickly than their White counterparts. Could this difference in aging between Black and White Americans be due to racial disparities in neighborhood environments? Using survey responses and additional data on air pollution from the Environmental Protection Agency, Isabel concluded that the Black Americans surveyed had lower neighborhood socioeconomic resources, higher levels of social deprivation, and increased exposure to fine particulate matter (PM2.5, the same pollutant given off by wildfire smoke). Moreover, these factors did contribute to the difference in DNA methylation-based age between Black and White participants, suggesting that neighborhood discrimination and deprivation are associated with accelerated aging among Black Americans.
Interestingly, Isabel found differences not only in the amount of PM2.5 exposure between Black and White participants, but also in the extent to which this exposure to air pollution affected aging. In particular, analysis revealed that Black individuals appeared more vulnerable to the effects of air pollution. This increased vulnerability was a result of fewer socioeconomic resources, which impacts things like the proximity to major sources of pollution and the quality of air filtration available. In other words, the neighborhood environments of Black participants not only put them at a higher risk for exposure, but also made them more susceptible to exposure’s damaging effects.
This study has numerous important implications. For one, Isabel’s work provides evidence that working to eliminate the racial gaps in neighborhood social and economic conditions would go a long way toward alleviating the accelerated biological aging and increased vulnerability to environmental exposure present among Black Americans. It also highlights just how important information about neighborhood conditions can be for health and aging, inviting future exploration of how local environments contribute to our health across the lifespan.
About the brief writer: Kara McGaughey
Kara is a PhD candidate in Josh Gold’s lab studying how we make decisions in the face of uncertainty and instability. Combining electrophysiology and computational modeling, she’s investigating the neural mechanisms underlying this adaptive behavior.
Citations:
Diez Roux, A. V. (2016). Neighborhoods and Health: What Do We Know? What Should We Do? American Journal of Public Health, 106(3), 430–431. https://doi.org/10.2105/AJPH.2016.303064
Tessum, C. W., Apte, J. S., Goodkind, A. L., Muller, N. Z., Mullins, K. A., Paolella, D. A., Polasky, S., Springer, N. P., Thakrar, S. K., Marshall, J. D., & Hill, J. D. (2019). Inequity in consumption of goods and services adds to racial–ethnic disparities in air pollution exposure. Proceedings of the National Academy of Sciences, 116(13), 6001–6006. https://doi.org/10.1073/pnas.1818859116
Lennon, J. C., Aita, S. L., Bene, V. A. D., Rhoads, T., Resch, Z. J., Eloi, J. M., & Walker, K. A. (2022). Black and White individuals differ in dementia prevalence, risk factors, and symptomatic presentation. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association, 18(8), 1461–1471. https://doi.org/10.1002/alz.12509
Diez Roux, A. V. (2016). Neighborhoods and Health: What Do We Know? What Should We Do? American Journal of Public Health, 106(3), 430–431.https://doi.org/10.2105/AJPH.2016.303064
Interested in reading more? You can check out Isabel’s paper here!
Sex matters: Exploring sex differences in opioid withdrawal mechanisms
or technically,
Sex differences in VTA GABA transmission and plasticity during opioid withdrawal
[See original abstract on Pubmed]
Dan Kalamarides was the lead author on this study. Dan’s research interests are rooted in neuropsychopharmacology, that is, the intersection of brain physiology, drugs (both “good” and “bad”), and behavior. This interest has been applied in the context of preclinical models for several therapeutic areas including substance use disorders, pain, neuroinflammation, and depression. Dan is currently planning to transition to industry where he can leverage his neuroscience expertise in the pharmaceutical world to enhance treatment strategies for mental health disorders and brain diseases.
or technically,
Sex differences in VTA GABA transmission and plasticity during opioid withdrawal
[See Original Abstract on Pubmed]
Authors of the study: Daniel Kalamarides, Aditi Singh, Shannon Wolfmann, John Dani
Scientific research has long been biased on the basis of sex. From cells and tissues to animals and people, there is a long history of scientists including more male subjects in their studies. As a result, we don’t understand how female bodies respond differently to diseases or to treatments, and the quality of healthcare has suffered. The National Institutes of Health (NIH) and several scientific journals have started requiring researchers to consider sex in their science, but the progress towards equal representation of males and females has been slow.
Opioids - including heroin, fentanyl, morphine, and others - are one of many classes of drugs that affect men and women differently. For example, women are less responsive to the pain killing effects of opioids but more sensitive to affects the drugs have on respiration compared to men. This difference makes it a lot harder to safely and effectively treat women with opioids in the clinical setting, and it can make recreational opioid use more dangerous. Despite these differences in people, basic science research into the effects and mechanisms of opioids in females is still lacking compared to our understanding of the drugs in males.
One area of research on opioids that still has a lot of unanswered questions, related to sex differences and more generally, is opioid withdrawal. Scientists, including recent NGG graduate Daniel Kalamarides, want to better understand opioid withdrawal so that they can treat the withdrawal, help people feel better, and make it easier for people to stop using opioids. In his paper, Daniel and his fellow researchers wanted to learn more about how the brain changes during opioid withdrawal, while keeping in mind that these changes could look different in males and females. Specifically, he was curious about a brain region called the ventral tegmental area (VTA), which contains neurons responsible for releasing dopamine into another brain region (the striatum) involved in reward.
Previous studies have shown that the active effects of opioids (think the “high”) are in part caused by an increase in dopamine release from those neurons in the VTA. This happens because opioids remove a natural brake on the dopamine system. In an opioid-free brain, other inhibitory neurons in the VTA – known as GABAergic neurons because they release the neurotransmitter GABA – decrease the release of dopamine from the dopaminergic neurons. Opioids remove this brake by decreasing the activity of the inhibitory neurons. This makes the system go faster, or, more specifically, release more dopamine.
Your brain adapts if opioids are in the body for an extended period of time. In the VTA, this means that those inhibitory neurons amp up their control of the dopamine-releasing neurons so that, even in the presence of an opioid, a relatively normal amount of dopamine is released. This is fine until the opioids are removed. Now you have an overactive brake, and there’s not enough dopamine released into the reward-related brain regions.
Researchers have found, in male mice only, that the inhibitory neuron control of the dopamine-releasing neurons increases in withdrawal because the connections between them grow stronger. This increase in connectivity is known as long term potentiation (LTP) or plasticity, and it’s one of the primary mechanisms by which the brain changes depending on how it’s used and what it’s exposed to. Knowing that the effects of opioids can differ between males and females, Daniel explored whether a similar phenomenon occurs in female mice.
Daniel first induced opioid withdrawal in mice by giving them morphine for a week, then studied the properties of neurons in the VTA when the mice were in withdrawal. He used patch-clamp electrophysiology, a technique which allowed him to measure the electrical current flowing into or out of the neuron as he manipulated the voltage. By using this technique, he was able to learn about the strength of the connection between the inhibitory neurons and the dopaminergic neurons and compare that connection between male and female mice.
Daniel measured how likely the inhibitory neurons were to release GABA – and thus inhibit the dopamine-releasing neurons – spontaneously and when electrically stimulated. He found that, in male mice, morphine withdrawal increased the probability of GABA release (or increased the strength of that brake). This was a great result because previous studies had also found this phenomenon, which means that this science is replicable. When he looked at female mice, however, he didn’t see any difference between the morphine treated mice and the control mice. That’s a surprise!
Daniel also tried to experimentally force LTP to occur in the brains in morphine withdrawal so that he could learn more about how the probability of GABA release was changing. He stimulated the inhibitory neurons with a really high frequency of electrical current, which would cause LTP in a normal neuron. He found that he could cause plasticity in the female mice, but he couldn’t in the males. This result suggested that the increase in the probability of GABA release in males was due to LTP. The molecular components needed for LTP were all used up in the males, so Daniel couldn’t create more. The components were still available in the females, on the other hand, so Daniel was able to stimulate the neurons and cause LTP.
To be thorough, Daniel also asked if the male and female mice were experiencing a similar level of morphine withdrawal. If the female mice were going through less withdrawal, it could maybe explain the sex differences in plasticity in the VTA. Daniel measured the strength of withdrawal that the mice were experiencing by counting physical signs of morphine withdrawal, and he found that males and females displayed a similar number. After all of these experiments, we still don’t know for sure that the opioid withdrawal mechanisms in male and female mice are entirely different. If Daniel used a different dose of morphine or if he studied the brains at a different time into withdrawal, he might be able to observe the same plasticity in female mice that he saw in male mice. However, by running this control experiment, he was able to strengthen the argument that there is a true difference in how the male and female mouse brains changed in opioid withdrawal.
This research by Daniel and his fellow scientists reinforced the fact that opioids affect males and females differently, and they showed that we still don’t understand how female brains change in opioid withdrawal. Hopefully, this evidence will push other scientists to continue thinking about sex differences in opioid research and in neuroscience broadly. In the meantime, Daniel has led us a step closer towards developing treatments for opioid use disorder, and he’s contributed to reducing bias in science.
About the brief writer: Lyndsay Hastings
Lyndsay is a first year NGG PhD student broadly interested in the relationship between neurocircuitry and behavior.
Interested in learning more about how opioid withdrawal is different in males and females? Check out Daniel’s paper here!
Weight loss drugs can also be leveraged to curb nicotine use
or technically,
Liraglutide attenuates nicotine self-administration as well as nicotine seeking and hyperphagia during withdrawal in male and female rats
[See original abstract on Pubmed]
Rae Herman was the lead author on this study. Rae is a 5th year PhD Candidate in the lab of Dr. Heath Schmidt. Her research investigates the potential of repurposing current medications for obesity/type II diabetes as novel treatments for substance use disorders. She also explores neural basis of drug seeking with a focus on nicotine and fentanyl use disorder.
or technically,
Liraglutide attenuates nicotine self-administration as well as nicotine seeking and hyperphagia during withdrawal in male and female rats
[See Original Abstract on Pubmed]
Authors of the study: R J Herman, M R Hayes, J Audrain-McGovern, R L Ashare, & H D Schmidt
Nicotine use has long plagued humanity, despite how it negatively impacts our health. Indeed, numerous studies have shown that daily smoking leads to increased risk of cancers, lung disease, and heart disease1. However, despite this knowledge, most nicotine users are unable to stop smoking over the long-term. Only 10% of smokers are successful when they try to quit smoking2, and individuals who do manage to quit often develop strong cravings for highly palatable foods (i.e., foods that taste sweet or savory with high fat and/or sugar content)3. Weight gain during nicotine abstinence is a major concern for people trying to quit smoking and is one of the most significant barriers to quitting for good4. Current approved treatments for nicotine use, like varenicline and bupropion, are only modestly effective and do not prevent weight gain after quitting5.
However, there might be a pharmacological therapy that provides some hope for nicotine addiction. A class of drugs known as glucagon-like peptide-1 receptor (GLP-1R) agonists have recently been approved by the FDA to treat obesity. These drugs have been tremendously successful, with individuals suffering from obesity losing approximately 20% of their weight after long-term use6. The success of the drug has captured the attention of many people. For example, there are numerous ads on TV or social media posts relaying stories of people using the GLP-1R-based drugs for weight loss. Science magazine even named the development of this drug class as the “Breakthrough of the Year” in 20237. With the success of these drug treatments, users noticed that they had reduced cravings for both palatable foods and drugs such as nicotine and alcohol8.
This report was intriguing to Dr. Heath Schmidt, a professor at the University of Pennsylvania (Penn). His team was led by Rae Herman, a graduate student in the Neuroscience Graduate Group at Penn. They hypothesized that this class of miracle weight loss drugs might reduce nicotine use, and simultaneously prevent the cravings for highly palatable foods that arise after quitting smoking. Effectively, using one drug to treat two conditions, or essentially using one stone to kill two birds.
To test their hypothesis, the researchers used a rodent model of drug taking. Specifically, they inserted an intravenous catheter into the jugular vein of rats. By doing this, they could train the rats to press a lever and receive an infusion of nicotine into the bloodstream. The rats repeatedly self-administer nicotine, which mimics nicotine use throughout the day in humans. Once the rats became addicted to nicotine, the experimenters treated half of the mice with the GLP-1R agonist liraglutide, and the other half of the mice received no treatment. Strikingly, after mice were treated with liraglutide, they had a reduced number of self-administered infusions for nicotine. They also demonstrated less nicotine seeking lever presses in reinstatement tests, an animal model of relapse to drug taking. These results provide evidence that GLP-1R drugs can be repurposed from their current use as weight loss drugs to reduce nicotine use and relapse.
Next, the researchers evaluated how nicotine withdrawal affects food intake and body weight. First, rats self-administered nicotine every day for 21 days. Then, the rats went through nicotine withdrawal, where they no longer had access to nicotine. During withdrawal, the rats were given a highly palatable food source, which was high in fat and sugar. During nicotine withdrawal, rats ate more of the highly palatable food and gained more weight than a control group of rats that had no nicotine experience. This experiment confirmed that rats, like humans, eat more of highly palatable foods and gain weight during nicotine withdrawal. In parallel with this experiment, a separate group of nicotine-addicted rats received daily treatment with GLP-1R agonist liraglutide during the nicotine withdrawal period. Strikingly, although these rats were also given the highly palatable food, they consumed less food and did not gain weight compared to control rats that had no nicotine experience. Therefore, liraglutide was able to stop nicotine withdrawal from causing increased palatable food intake and body weight gain.
This exciting research suggests a novel role for GLP-1R-based drugs to curb nicotine use and prevent nicotine withdrawal-induced weight gain. Future studies can now determine the effectiveness of GLP-1R drugs at reducing nicotine use in humans, and if other drugs can be leveraged, in combination with GLP-1R drugs, to further help nicotine users achieve long-term abstinence.
About the brief writer: Aaron McKnight
Aaron is a PhD Candidate in Amber Alhadeff’s lab. The Alhadeff Lab is focused on understanding how different macronutrients are detected within the gastrointestinal tract and how this information is relayed to hypothalamic agouti-related protein (AgRP)-expressing neurons (a group of neurons that drive feeding behavior!).
Citations:
1. CDC (2020) Smoking Cessation: A Report of the Surgeon General. CDC Center for Disease Control and Prevention.
2. Babb S, Malarcher A, Schauer G, Asman K, Jamal A (2017) Quitting Smoking Among Adults - United States, 2000-2015. MMWR Morb Mortal Wkly Rep 65:1457–1464
3. Chao AM, Wadden TA, Ashare RL, Loughead J, Schmidt HD (2019) Tobacco Smoking, Eating Behaviors, and Body Weight: A Review. Curr Addict Rep 6:191–199
4. Benowitz NL (2009) Pharmacology of nicotine: addiction, smokinginduced disease, and therapeutics. Annu Rev Pharmacol Toxicol 49:57–71
5. Mills EJ, Wu P, Lockhart I, Thorlund K, Puhan M, Ebbert JO (2012) Comparisons of high-dose and combination nicotine replacement therapy, varenicline, and bupropion for smoking cessation: a systematic review and multiple treatment meta-analysis. Ann Med 44:588–597
6. Wilding JPH, Batterham RL, Calanna S, Davies M, Van Gaal LF, Lingvay I, McGowan BM, Rosenstock J, MTD T, Wadden TA, Wharton S, Yokote K, Zeuthen N, Kushner RF, Group SS (2021) Once-Weekly Semaglutide in Adults with Overweight or Obesity. N Engl J Med 384:989
7. Couzin-Frankel, J. Obesity Meets Its Match. Science (2023). Vol 382, Issue 6676.
8. Blum, D. People on Drugs Like Ozempic Say Their “Food Noise” Has Disappeared. (2023) New York Times.
Understanding the brain during mindfulness
or technically,
Mindful attention promotes control of brain network dynamics for self-regulation and discontinues the past from the present
[See original abstract on Pubmed]
Dale Zhou was the lead author on this study. Dale is interested in how the brain network compresses and reconstructs information as network structure changes across the lifespan. He aims to account for computations of memory and reward as network functions of dimensionality reduction and expansion using experimental, naturalistic, and clinical data.
or technically,
Mindful attention promotes control of brain network dynamics for self-regulation and discontinues the past from the present
[See Original Abstract on Pubmed]
Authors of the study: Dale Zhou, Yoona Kang, Danielle Cosme, Mia Jovanova, Xiaosong He, Arun Mahadevan, Jeesung Ahn, Ovidia Stanoi, Julia K. Brynildsen, Nicole Cooper, Eli J. Cornblath, Linden Parkes, Peter J. Mucha, Kevin N. Ochsner , David M. Lydon-Staley, Emily B. Falk, and Dani S. Bassett
In recent years, the practice of meditation has received a lot of attention for its health benefits, both physically and mentally. One popular form of meditation, mindfulness meditation, teaches individuals to focus on, and attend to the present moment. The ability to shift focus depends on the ability to orchestrate shifts in neural activity, and has been previously called executive function. While the benefits of mindfulness meditation are widely recognized, what’s going on in the brain is much less clear.
In order to understand how mindfulness is represented in the brain, Dale Zhou, a recent NGG graduate, and his collaborators recruited healthy college students who identified as social drinkers and asked them to perform a task rating from 1 to 5 how much they would crave an alcoholic drink, presented to them on a computer screen. Dale simultaneously measured the activity patterns in participants’ brains using functional magnetic resonance imaging, or fMRI, while they completed this task. One group of participants was instructed to practice mindfulness while rating their cravings by “mentally distancing themselves by observing the situation and their response to it with a more impartial, nonjudgmental, or curious mindset, and without getting caught up in the situation or response”. The other group was instructed to rate their cravings with their natural gut reaction to the drink. For some trials, participants in the mindful group were asked to switch to their gut reaction instead, allowing Dale and his colleagues to compare which brain areas were simultaneously active or quiet during the different reactions. This allowed them to draw some interesting conclusions about how the brain represents mindfulness.
Figure 1: Simplified representation of brain states. In this example, the brain has only two areas and the brain state is defined by the activity of region 1 and region 2.
Before Dale analyzed the results from the experiment, he first asked how mindfulness can be measured in the brain and if the “amount” of mindfulness in our brains impacts our day-to-day behaviors. To answer this question, he used average brain activity from the participants’ scans to calculate a measure of the executive function called controllability. To understand controllability, it is helpful to think of the brain as having different “brain states” (Figure 1). When a person is doing some activity, like walking, the brain exists in a particular brain state - some brain areas are very active and some are quiet. When the same person is doing a different activity, like eating, the brain exists in a different brain state - a different set of brain areas are active and quiet. Dale and his colleagues defined controllability as how readily the brain can switch into any possible brain state. By calculating controllability for each participant, and tracking their drinking behavior weeks after the brain scan, Dale found that the participants with higher controllability tended to have fewer drinks than those with lower controllability, suggesting that perhaps mindfulness does impact our day to day behaviors in a positive manner.
Now back to the experiment. Dale asked whether there were differences in controllability, and therefore brain activity, between the two groups. To do this, he calculated the amount of effort, or control, it took for participants in each group to enter either a mindful state or gut reaction state while reacting to the alcohol cue. He found that participants instructed to react mindfully took more effort to enter this brain state after being prompted than participants instructed to react naturally took to enter their gut reaction brain state. This was exactly what they expected to see, since it is known that achieving a state of mindfulness initially requires more thought and brain activity. However, he also found that when participants from both groups were instructed to react naturally, those who had previously reacted mindfully still required more effort to enter this gut reaction brain state than those who had not. This suggests that practicing mindfulness might make us more effortful in attention, even when we are not actively trying or instructed to.
Finally, Dale found that brain areas that use more effort had shorter episodes of neural activity. These shorter episodes suggested that there was less influence of the past in these areas. Furthermore, these quick episodes were typically found in brain areas that help us sense the world around us rather than areas that help us think about past experiences or plan for the future. Practicing mindfulness, therefore, may put us in a more effortful state of attention which is more focused on the present moment rather than on the past or future.
In conclusion, Dale’s hard work on this project has allowed us to take a glimpse at the brain during mindfulness and how it might be benefiting our behavior. His work reminds us that, although the brain is composed of many different brain areas, human behavior is a product of these various areas interacting with one another, producing unique states of mind such as mindfulness. Work similar to his will hopefully lead the way to a better understanding of some of the brain’s other complex functions.
About the brief writer: Jafar Bhatti
Jafar is a PhD Candidate in Long Ding and Josh Gold’s lab. He is broadly interested in brain systems involved in sensory decision-making.
Want to learn more about how these researchers study mindfulness? You can find Dale’s paper here!
A new method for looking through the (cyto)skeletons in the closet
or technically,
A solution to the long-standing problem of actin expression and purification
[See original abstract on pubmed]
Rachel Dvorak was the lead author on this study. She is interested in studying the biochemical mechanisms by which mutations in gamma smooth muscle actin cause visceral myopathy. Patients with visceral myopathy present with severe abdominal distension, intractable constipation, feeding intolerance, and growth delays. Because there are no targeted therapies for this disease, many patients die in adolescence. She hopes that by understanding how disease-causing mutations alter actin biochemistry we can develop treatments for patients with visceral myopathy and other rare conditions caused by actin mutations.
or technically,
A solution to the long-standing problem of actin expression and purification
[See Original Abstract on Pubmed]
Authors of the study: Rachel H. Ceron, Peter J. Carman, Grzegorz Rebowski, Malgorzata Boczkowska, Robert O. Heuckeroth, and Roberto Dominguez
Take a moment to picture the last evening you spent with friends. Now, think about something that happened over 10 years ago.
Throughout both of those events- in fact, throughout every day of your life- you’ve had the same exact cells living in your brain, helping you navigate each situation. These cells are called neurons, and your body can’t replace them; when they’re gone, they’re gone for good. This means it’s especially important for neurons to be well-built to withstand the everyday challenges that cells face, and well-equipped to respond to emergencies that could threaten their existence.
One contributor to neurons’ ability to last a lifetime lies in how they’re built; much like buildings that are constantly exposed to the elements, neurons need structural integrity to last a lifetime. To do this, there are several types of miniature “skeletons” that exist within the cell. Making up these miniature cellular skeletons, collectively called the cytoskeleton, are proteins. Proteins are large molecules that perform specific jobs intended to keep cells alive. One type of protein is called actin. Similar to links in a chain, actin proteins can attach to each other to form a long structure called a filament. As part of the cytoskeleton, actin filaments are important for helping neurons and many other types of cells keep their shape. Correspondingly, neurons are only as structurally sound as their components; issues within actin filament structures cause severe defects in overall brain structure. This includes lissencephaly, a condition where the brain lacks the grooves that normally sprawl across its surface, leaving it completely smooth. By understanding how actin behaves, both on its own and in the presence of other proteins, scientists hope to develop better treatments for diseases where actin isn’t acting the way it normally would.
Rachel Dvorak, a PhD candidate in Dr. Roberto Dominguez’s lab at the University of Pennsylvania, wanted to develop a robust way to study how actin works. Specifically, she was interested in studying how actin interacts with itself and other proteins- much like people, proteins can interact with each other in ways that influence their behavior in the cell. To do this, she aimed to isolate actin from cells. Isolating a single type of protein is a common method to study the way it functions. The inside of a cell is a bustling place, and it can be hard to tease out the specific interactions that proteins have with one another when they exist within that cellular environment.
This is far from a simple undertaking. Inside of an actual cell, there are many actin proteins, and they are not all identical. Instead, there are several possible varieties of actin. One way to understand this is by comparing it to different flavors of ice cream. Although chocolate and vanilla are made of slightly different ingredients and paired with different foods, they are both still ice cream. Actin also comes in different “flavors” called isoforms. While each of their structures are slightly different, and each isoform might be best suited for use in different scenarios, they are all still considered actin due to their overall similarities and jobs in the cell. Moreover, even two actin proteins that are the same isoform can be slightly different, because the cell can modify actin by attaching other molecules to it. These attachments are called post-translational modifications, and they also influence actin’s behavior. However, despite their important effect on actin behavior, many of these post-translational modifications are usually lost during the process of isolating actin to study its behavior.
Another challenge of isolating specific isoforms of actin lies in getting cells to produce large quantities of the actin isoform of interest. Making actin is an involved, multi-step process for the cell that requires a lot of molecular machinery. Because of this, most scientists use only one type of actin isolated from muscle cells to conduct experiments outside of the cellular environment. This severely limits our ability to study whether different isoforms of actin behave in slightly different ways. It also means that oftentimes, our out-of-the-cell reconstructions of cellular events are created using actin filaments that lack much of the nuance they would have in cells. This limits the accuracy of this model and makes it impossible to study how actin that is incorrectly produced causes human disease.
To tackle this, Rachel decided to use modified human kidney cells to produce actin. This meant that the cells would already contain the machinery unique to humans that is necessary to carry out production of actin. This is in contrast with existing methods that use cells like bacteria or insect cells; these cell types can also be used to churn out proteins for isolation, but lack the machinery to make some proteins native to human cells or add post-translational modifications the way that human cells would. Rachel was able to introduce genetic instructions that caused the human kidney cells she worked with to essentially become actin-making factories, synthesizing large quantities of whichever actin isoform she was interested in.
However, the actin the kidney cells made based on the genetic instructions that Rachel provided was a bit different than any other actin in the cells; each of these actin proteins was also attached to two other man-made proteins called tags. Tags are helpful because scientists have materials that can grab onto them, allowing for the separation of the tags (and anything attached to them) from everything else in the cell. Most tags don’t occur naturally, so only the protein (or, in this case, the actin isoform) that you’ve instructed the cell to produce will have this unique feature. To further ensure she was isolating only the type of actin she wanted, Rachel also used a different protein her lab had engineered to grab and release actin based on how much calcium is in the environment. By using a combination of materials that grab the tags attached to actin in tandem with the protein that grabs onto actin itself, Rachel was able to isolate specific isoforms of actin with post-translational modifications from the rest of the contents of the kidney cells.
Rachel and her colleagues ultimately invented a new method for isolating actin exactly as it would exist in the cell. This is important because it means scientists now have a new way to study the exact things that are going awry in diseases that involve issues with interactions between actin and other proteins, such as the one that causes lissencephaly. The more we understand about how actin functions differently in diseases like this, the better our ability to develop effective treatments.
About the brief writer: Julia Riley
Julia is a PhD candidate in Dr. Erika Holzbaur’s lab studying the consequences that damaged mitochondria (the powerhouses of the cell) have on the function of astrocytes, a cell type found in the brain. This is important for understanding diseases like Parkinson’s, where we know mitochondrial damage occurs but don’t fully understand how it impacts the health of brain cells.
Interested in reading more about actin? Find the full paper here!
Little kids, big insights: What childhood can teach us about how the brain supports cognition
or technically,
The age of reason: Functional brain network development during childhood
[See original abstract on Pubmed]
Ursula Tooley was the lead author on this study. Ursula is a postdoctoral research scholar at Washington University in St. Louis. Her research examines functional brain network development in neonates and toddlers, with a focus on the pace of brain maturation and how neuroplasticity changes across development. She received her Ph.D. in Neuroscience in 2022 from the University of Pennsylvania, under the direction of Dr. Allyson Mackey and Dr. Dani Bassett, where she studied functional brain network development in children and adolescents. She received her B.S. in Neuroscience from the University of Arizona, where she conducted research on sleep disruption in children with Down syndrome.
or technically,
The age of reason: Functional brain network development during childhood
[See Original Abstract on Pubmed]
Authors of the study: Ursula A. Tooley, Anne T. Park, Julia A. Leonard, Austin L. Boroshok, Cassidy L. McDermott, Matthew A. Tisdall, Dani S. Bassett, and Allyson P. Mackey.
Early and middle childhood (4-10 years old) are full of developmental milestones. How children speak, move, learn, and play is constantly evolving and improving. Kids build social networks, become better able to control their attention, and begin to develop cognitive skills, like reasoning. However, despite the rapid cognitive development happening during this early childhood period, neuroscientists have very little information about how brain function is changing.
This is because getting a clear picture of brain activity, like getting a clear picture of anything, requires that the subject stays almost perfectly still. If you’ve ever watched a 4-year-old sit at the dinner table, it comes as no surprise that they don’t make the best neuroimaging subjects. Functional magnetic resonance imaging (fMRI) scanners, which take many consecutive snapshots of brain activity, are even more sensitive to motion than cameras. The tiniest movements, even just a few millimeters, can blur the images and make it impossible for neuroscientists to tell what brain activity belongs to which brain region. So, most neuroimaging work to date uses subjects over 7 years old, which means that while researchers work to understand how the brain develops to support cognition, they’re missing many of the first pieces of the puzzle.
Here’s where recent Neuroscience Graduate Group alumn Ursula Tooley and collaborators from the Robust Methods for Magnetic Resonance group stepped in. The team engineered a way to monitor and correct for head motion inside the scanner, allowing them to collect high-quality neuroimaging data from wiggly subjects during this critical early childhood period. Specifically, this motion-tracking technology gave the researchers a way to record exactly how much and in which direction kids were moving at any given point during the scan. Ursula could then use this information to correct (think: realign) the images of brain activity or exclude the child from the study if they moved too much. The ability to precisely monitor head position in real time also created an opportunity for kids to practice the correct behavior. Before the scanning session, children came to the lab to watch a movie while laying in a mock scanner that made the same whirring noises and beeps as the real deal. Each time they moved their head more than 1 millimeter, the movie paused. Incorporating this period of exploring the scanner and the scanning expectations meant that most of the kids who enrolled in the study stayed still enough for usable images of brain activity to be collected. This is a huge feat for Ursula and the team as well as a huge win for neuroscience, making it possible to take an earlier look at the developing brain.
Over the course of the study, Ursula and her colleagues scanned a diverse group of 92 children ages 4-10 from the Philadelphia community. Each child completed an fMRI scan as well as a series of cognitive tests (which they did outside of the scanner) designed to measure the strength of their cognitive reasoning abilities. What is cognitive reasoning? Reasoning is an umbrella term describing the ability to process information, problem solve, and make predictions based on pattern recognition (Fig. 1). Successful cognitive reasoning involves much of the brain and improves dramatically during early and middle childhood. Research suggests that how kids perform on cognitive reasoning tasks is predictive of their academic achievement — even years down the road! By combining a child's cognitive reasoning ability with information about their brain activity, Ursula was able to ask whether and how changes in brain function might support this shift in cognitive performance.
Figure 1
An example question from the cognitive reasoning test, which was administered at different difficulty levels to children in the study depending on their age. Here, we see the red rectangle switches from the background (left) to the foreground (right). To answer the question correctly, the child has to understand this spatial relationship for the rectangles and extend it to the pentagons.
Ursula used resting-state fMRI data (data collected while the kids laid “at rest” in the scanner) to explore the brain’s functional organization. In other words, she inferred how much different brain regions talk to each other based on how their activity fluctuates together over time. As such, regions with activity that rises and falls together are likely functionally connected. These groups of connected brain regions are called “systems.” The brain has many of these functionally-connected systems, and neuroscience research shows that they can rewire and reconfigure themselves. For example, another neuroimaging study of older kids and young adults (ages 8-22) from Philadelphia showed that the organization of these brain systems changes with age [1]. Specifically, our brain systems become more segregated and more modular as we move towards adulthood, with weaker connections between systems and stronger connections within systems (Fig. 2). Ursula found the same trends in her data with older kids tending to have more segregated brain systems than younger kids, suggesting that our brain’s functional architecture is flexible and continues to refine as we age.
Figure 2
As we age and develop, our brain systems (red, green, and blue ovals) reorganize, moving from more integrated (e.g., many connections between systems) to more modular (e.g., more connections within systems and fewer connections between systems). Ursula’s work shows that this brain system separation supports the development of cognitive skills, like reasoning.
Do some systems remodel more than others? Ursula found that changes in connectivity were largest in brain systems involved in abstract cognition, visual processing, and attention. As it turns out, these are the same systems involved in cognitive reasoning. For instance, reasoning is supported by the brain’s visual areas taking in information from the world while attention systems focus the brain’s resources on what’s important to the task at hand while ignoring distractors. Given what we know about the blossoming of cognitive reasoning during childhood, Ursula wondered if there could be a relationship between these changes in brain connectivity and cognitive ability. To test this, Ursula compared the patterns of brain system connectivity for each child with their scores on the cognitive reasoning test (Fig. 1). She found that the remodeling of cognition, visual processing, and attention systems was associated with increased cognitive ability! In other words, kids who had more mature patterns of brain system connectivity were better equipped to reason about the world and their place in it.
Taken together, Ursula’s work suggests that the massive restructuring of brain systems as kids age might be happening to support the rapid development of cognitive abilities emerging during these early and middle childhood years. Beyond offering a new perspective on healthy brain development, this relationship between brain organization and brain function offers new ways to think about -- and potentially treat -- various neurodevelopmental or neurological disorders.
About the brief writer: Kara McGaughey
Kara is a PhD candidate in Josh Gold’s lab studying how we make decisions in the face of uncertainty and instability. Combining electrophysiology and computational modeling, she’s investigating the neural mechanisms that may underlie this adaptive behavior.
Citations:
Baum, G.L., Ciric R., Roalf, D.R., Betzel, R.F., Moore, T.M., Shinohara, R.T., … & Satterthwaite, T.D. (2017). Modular segregation of structural brain networks supports the development of executive function in youth. Current Biology, 27(11). doi: 10.1016/j.cub.2017.04.051.