BRAINS IN BRIEFS
Scroll down to see new briefs about recent scientific publications by neuroscience graduate students at the University of Pennsylvania. Or search for your interests by key terms below (i.e. sleep, Alzheimer’s, autism).
Why some people wake up under anesthesia and others don’t (Hint: It’s your Hormones)
or technically,
Hormonal basis of sex differences in anesthetic sensitivity
[See original abstract on Pubmed]
Dr. Andrzej (Andi) Wasilczuk was the lead author on this study. A recent graduate from Penn Bioengineering, Andi is captivated by the brain’s intricate ability to shift between states of consciousness. His research uses general anesthetics to uncover the neuronal circuits responsible for these shifts, aiming to understand how the brain sustains or disrupts consciousness. By identifying these critical networks, Andi is paving the way for personalized anesthesia and offering new insights into arousal state transitions.
or technically,
Hormonal basis of sex differences in anesthetic sensitivity
[See Original Abstract on Pubmed]
Authors of the study: Andrzej Z Wasilczuk, Cole Rinehart, Adeeti Aggarwal, Martha E Stone, George A Mashour, Michael S Avidan, Max B Kelz, Alex Proekt, ReCCognition Study Group
Waking up during surgery sounds like a nightmare, and did you know that females might be at higher risk for this than males? Through medication, general anesthesia makes a patient unconscious, which allows doctors to perform surgical procedures without the patient’s awareness or discomfort. General anesthesia puts the patient in a sleep-like state, and doing this is an involved process. Anesthesiologists must be highly trained to determine the best course of treatment. When creating a safe treatment plan, anesthesiologists take into account many factors, such as the patient’s body weight or pre-existing conditions. The sex of the patient, however, hasn’t been historically considered as an equally important factor in delivering a safe course of anesthesia.
Previous research about the link between sex and response to anesthesia was ambiguous and conflicting. Some early clinical trials suggested that females were more likely to wake up under anesthesia, while others found no significant difference between males and females. These clinical trials, however, had diverse patient populations and non-standardized anesthetic protocols, which would make it hard to directly compare anesthetic conditions between patients. Nevertheless, more recently, an analysis done on many of these studies has provided clear cut evidence that females are more resistant to the anesthetic state (Braithwaite et al., 2023). The question of how this sex difference arose, however, remained unanswered.
Dr. Andi Wasilczuk, a former Penn Bioengineering PhD student, and his team wanted to understand why females and males responded differently to anesthesia. To do this, they decided to focus on the hypothalamus, a structure in the brain heavily involved in both sleep-wake and anesthetic induced unconsciousness. The hypothalamus is regulated by hormones, which are the body’s chemical messengers—and the researchers knew that the levels of hormones typically differ between males and females. For example, males typically have a much higher level of the hormone testosterone, whereas females typically have higher levels of the hormone estrogen.
With these differences in mind, Dr. Wasilczuk wanted to know: Could hormonal differences across sexes alter the effectiveness of general anesthesia? He framed "effectiveness of general anesthesia" by using the idea of “anesthetic sensitivity.” Individuals who are more sensitive to anesthesia need less of the drug to fall and stay unconscious, and wake up smoothly after surgery. On the contrary, individuals with less anesthetic sensitivity, or have anesthetic resistance, require more anesthetic to fall and stay asleep, and wake up sooner once the anesthetic is removed.
Recognizing this gap in the research, Dr. Wasilczuk’s research group sought to test the influence of sex and sex hormones on anesthetic sensitivity in mice. First, the researchers compared the dosage of anesthetic required for the mice to be initially anesthetized (induction), and to wake up from anesthesia (emergence). They found that, across all four anesthetics the group tested, female mice required a much higher dose on induction, and were more likely to emerge at higher doses than males. Next, the researchers compared the time, given the same dosage, for female versus male mice to be induced and emerge from anesthesia. Female mice took significantly longer to be induced than males, and also were much quicker to emerge. These experiments indicated that female mice were indeed more resistant to anesthesia, compared to male mice.
Yet, the reason for these results remained unclear: Were these effects due to sex hormone differences? To find out, the researchers changed the mice's hormone levels by surgical removal of the testicles (castration) in male mice or ovaries (oophorectomy) in female mice post puberty. They repeated the experiments, this time using castrated males and oophorectomy females, then compared these mice to the untreated males and females tested before.
The results were striking. In both experiments, castrated males and oophorectomized females showed a similar resistance to anesthesia as untreated females. Oophorectomy did not change a female mouse’s anesthetic sensitivity. Castration, however, produced a female-like anesthetic sensitivity in males. Eliminating male sex hormones, therefore, seemed to remove the sex differences in response to anesthesia!
The researchers also directly measured the effect of testosterone. Under a steady dose of anesthetic, untreated males and castrated males were injected with testosterone, and continually tested for responsiveness using the righting reflex. Testosterone administration increased anesthetic sensitivity for both groups of mice in a dose-dependent manner. This finding could explain why males, who typically have higher testosterone, are more sensitive to general anesthetics, and therefore are at lower risk of waking up under anesthesia than females.
Intrigued, the researchers wondered: Can these sex differences be seen in brain activity? The conventional measure of anesthetic depth (how unconscious someone is in response to anesthesia) during surgery is the Electroencephalogram (EEG). EEG measures electrical brain activity through electrodes attached to the scalp. The researchers found that sex differences were not reflected in the EEG of the mice they tested. Similar conclusions were made when re-analyzing human data from another study. In this study, female volunteers displayed resistance to general anesthesia based on assessments of behavior and cognitive function, but not based on information gathered from the EEG.
Looking at the activity of individual neurons, however, clearly revealed sex differences. They looked for elevated levels of the protein c-Fos, an indicator of neuronal activity, throughout the whole brain. Compared to anesthetized male mice, anesthetized female mice had fewer neurons expressing c-Fos in sleep-promoting hypothalamic cells. In other words, anesthesia activates fewer sleep-promoting circuits in females than males, correlating with females’ greater resistance to anesthetics.
Compared to untreated male mice, castrated male mice also had reduced c-Fos expression in similar hypothalamic structures. Fewer sleep-promoting circuits were activated in castrated males (which displayed a similar aesthetic sensitivity to females) than untreated males. Thus, sex-dependent activity patterns, seen in hypothalamic structures, reflected anesthetic sensitivity trends!
Dr. Wasilczuk’s groundbreaking paper reveals why researching sex-dependence is incredibly important: females may need different anesthetic management than males due to their higher resistance to anesthesia. After years of standard general anesthesia administration to millions of patients, and using EEGs to measure anesthetic depth, Dr. Wasilczuk’s findings have huge clinical implications supporting personalized anesthetic care.
About the brief writer: Sydney Liu
Sydney is a guest writer for Brains in Briefs! She is a Penn undergraduate in Dr. Shinjae Chung’s lab researching what makes us sleep and the brain transitions between sleep states. She is a Junior majoring in neuroscience, and is interested in teaching. In her free time, she likes to draw!
Citations:
E. Braithwaite et al., Impact of female sex on anaesthetic awareness, depth, and emergence: A systematic review and meta-analysis. Br. J. Anaesth. 131, 510–522 (2023).
Interested in learning more about how anesthetic sensitivity is different in males and females? Check out Andi’s paper here!
Neurons in the brainstem promote REM sleep and trigger brainwaves that might cause dreaming
or technically,
A medullary hub for controlling REM sleep and pontine waves
[See original abstract on Pubmed]
Dr. Amanda (Mandy) Schott was the lead author on this study. As a researcher, Mandy is most interested in defining neural circuits, and how specific populations of cells communicate to generate essential human behaviors such as sleep.
or technically,
A medullary hub for controlling REM sleep and pontine waves
[See Original Abstract on Pubmed]
Authors of the study: Amanda Schott, Justin Baik, Shinjae Chung & Franz Weber
Rapid eye movement (REM) sleep is the sleep state that most people associate with dreaming, however REM sleep has many other essential functions. While REM makes up only about 20-25% of our nightly sleep, it is vitally important for memory, emotional processing, and other functions we have yet to understand. This is true not just for humans, but all mammals and maybe even birds and reptiles! To facilitate all these functions of REM, the brain is highly active during this sleep state. In fact, during REM sleep, brain signals look more similar to wake than non-REM sleep. Because of this, REM sleep is sometimes called paradoxical sleep because paradoxically, the brain is so active during rest.
Surprisingly, we still know very little about how the brain switches from low-activity non-REM sleep to high-activity REM sleep. Moreover, during REM sleep there are sometimes sporadic brain waves that seem to be important for normal brain function but whose precise role is still not totally clear. P-waves are one such waveform that is caused by lots of synchronous neuronal activity in the back of the brain, in a brainstem region called the pons. From the pons, P-waves travel forwards in the brain to brain regions important for forming and storing memories, and also areas involved in visual processing. These P-waves are interesting because they occur only during REM sleep, and are proposed to be involved in dreaming and the memory functions of REM sleep. A paper by recent NGG graduate Dr. Amanda Schott investigated two major unknowns in REM sleep research: 1) What neurons and brain regions are involved in generating REM sleep, and 2) What neurons and brain regions are involved in generating P-waves. Is it possible that one set of neurons could do both?
While we know of several brain regions in the brainstem that regulate REM sleep, most of them consist of inhibitory neurons, meaning they “turn off’ other brain regions to promote REM sleep. Dr. Schott, however, found a highly unusual group of excitatory neurons in part of the brainstem called the dorsal medial medulla (dmM). These excitatory neurons can “turn-on” other neurons they make connections with. These dmM excitatory neurons were only active during REM sleep, suggesting they may be involved in promoting REMs sleep. In addition, dmM neurons project their axons and send signals to the part of the pons that is known to generate p-waves. In fact, the dmM neurons were active at the same time the p-waves occurred suggesting that the dmM excitatory neurons could be involved in the generation of p-waves too! Dr. Schott next wanted to directly manipulate the activity of these neurons to see if they could cause transitions to REM sleep or cause generate p-waves.
Using a modern neuroscience technique called optogenetics, Dr. Schott was able to cause the neurons in the dmM to fire when a laser light was shined over them through an optic fiber. She simultaneously determined if the mouse was awake, asleep, or in REM sleep by measuring the mouse’s brain waves using electroencephalography, or EEG. She found that stimulating these neurons caused the mouse to enter REM sleep, and also increased the length of REM sleep episodes. Shining the laser light also caused a p-wave to be generated when the light was shined about 60-100% of the time when the mouse was sleeping. Experimentally reducing the activity of the dmM neurons also decreased the amount of REM sleep, as well as the amount of p-waves. Dr. Schott interpreted these findings as evidence that dmM excitatory neurons are critical for normal amounts of REM sleep to occur, and for triggering p-waves.
Overall, Dr. Shott’s work adds an important piece to the puzzle to our understanding of which brain regions can promote REM sleep. Her findings are an important first step in understanding which neurons generate p-waves which is ultimately necessary to understand p-wave function. This work will provide a foundation on which others (including the author of this piece!) can study the role of p-waves in REM sleep, and move closer to finally understanding how and why we dream.
About the brief writer: Emily Pickup
Emily is a 4th year PhD candidate in Dr. Franz Weber’s lab. She is interested in the biological functions of sleep. Specifically, she is interested in understanding the function of REM-specific p-waves. The large pontine waveform implicated in memory consolidation discussed in the brief above.
Interested in learning more about REM sleep and p-waves? See the original paper here.
Understanding the brain during mindfulness
or technically,
Mindful attention promotes control of brain network dynamics for self-regulation and discontinues the past from the present
[See original abstract on Pubmed]
Dale Zhou was the lead author on this study. Dale is interested in how the brain network compresses and reconstructs information as network structure changes across the lifespan. He aims to account for computations of memory and reward as network functions of dimensionality reduction and expansion using experimental, naturalistic, and clinical data.
or technically,
Mindful attention promotes control of brain network dynamics for self-regulation and discontinues the past from the present
[See Original Abstract on Pubmed]
Authors of the study: Dale Zhou, Yoona Kang, Danielle Cosme, Mia Jovanova, Xiaosong He, Arun Mahadevan, Jeesung Ahn, Ovidia Stanoi, Julia K. Brynildsen, Nicole Cooper, Eli J. Cornblath, Linden Parkes, Peter J. Mucha, Kevin N. Ochsner , David M. Lydon-Staley, Emily B. Falk, and Dani S. Bassett
In recent years, the practice of meditation has received a lot of attention for its health benefits, both physically and mentally. One popular form of meditation, mindfulness meditation, teaches individuals to focus on, and attend to the present moment. The ability to shift focus depends on the ability to orchestrate shifts in neural activity, and has been previously called executive function. While the benefits of mindfulness meditation are widely recognized, what’s going on in the brain is much less clear.
In order to understand how mindfulness is represented in the brain, Dale Zhou, a recent NGG graduate, and his collaborators recruited healthy college students who identified as social drinkers and asked them to perform a task rating from 1 to 5 how much they would crave an alcoholic drink, presented to them on a computer screen. Dale simultaneously measured the activity patterns in participants’ brains using functional magnetic resonance imaging, or fMRI, while they completed this task. One group of participants was instructed to practice mindfulness while rating their cravings by “mentally distancing themselves by observing the situation and their response to it with a more impartial, nonjudgmental, or curious mindset, and without getting caught up in the situation or response”. The other group was instructed to rate their cravings with their natural gut reaction to the drink. For some trials, participants in the mindful group were asked to switch to their gut reaction instead, allowing Dale and his colleagues to compare which brain areas were simultaneously active or quiet during the different reactions. This allowed them to draw some interesting conclusions about how the brain represents mindfulness.
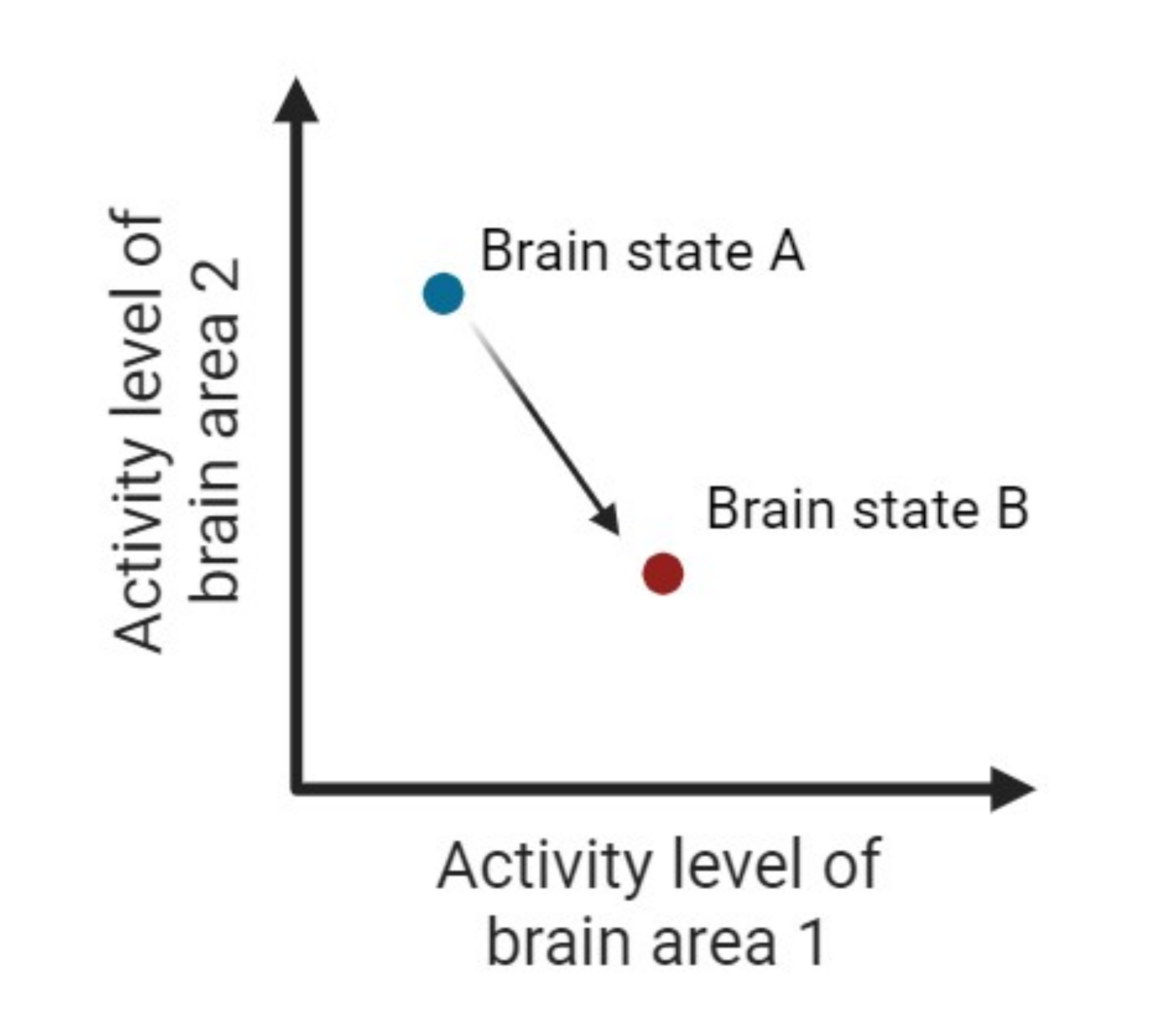
Figure 1: Simplified representation of brain states. In this example, the brain has only two areas and the brain state is defined by the activity of region 1 and region 2.
Before Dale analyzed the results from the experiment, he first asked how mindfulness can be measured in the brain and if the “amount” of mindfulness in our brains impacts our day-to-day behaviors. To answer this question, he used average brain activity from the participants’ scans to calculate a measure of the executive function called controllability. To understand controllability, it is helpful to think of the brain as having different “brain states” (Figure 1). When a person is doing some activity, like walking, the brain exists in a particular brain state - some brain areas are very active and some are quiet. When the same person is doing a different activity, like eating, the brain exists in a different brain state - a different set of brain areas are active and quiet. Dale and his colleagues defined controllability as how readily the brain can switch into any possible brain state. By calculating controllability for each participant, and tracking their drinking behavior weeks after the brain scan, Dale found that the participants with higher controllability tended to have fewer drinks than those with lower controllability, suggesting that perhaps mindfulness does impact our day to day behaviors in a positive manner.
Now back to the experiment. Dale asked whether there were differences in controllability, and therefore brain activity, between the two groups. To do this, he calculated the amount of effort, or control, it took for participants in each group to enter either a mindful state or gut reaction state while reacting to the alcohol cue. He found that participants instructed to react mindfully took more effort to enter this brain state after being prompted than participants instructed to react naturally took to enter their gut reaction brain state. This was exactly what they expected to see, since it is known that achieving a state of mindfulness initially requires more thought and brain activity. However, he also found that when participants from both groups were instructed to react naturally, those who had previously reacted mindfully still required more effort to enter this gut reaction brain state than those who had not. This suggests that practicing mindfulness might make us more effortful in attention, even when we are not actively trying or instructed to.
Finally, Dale found that brain areas that use more effort had shorter episodes of neural activity. These shorter episodes suggested that there was less influence of the past in these areas. Furthermore, these quick episodes were typically found in brain areas that help us sense the world around us rather than areas that help us think about past experiences or plan for the future. Practicing mindfulness, therefore, may put us in a more effortful state of attention which is more focused on the present moment rather than on the past or future.
In conclusion, Dale’s hard work on this project has allowed us to take a glimpse at the brain during mindfulness and how it might be benefiting our behavior. His work reminds us that, although the brain is composed of many different brain areas, human behavior is a product of these various areas interacting with one another, producing unique states of mind such as mindfulness. Work similar to his will hopefully lead the way to a better understanding of some of the brain’s other complex functions.
About the brief writer: Jafar Bhatti
Jafar is a PhD Candidate in Long Ding and Josh Gold’s lab. He is broadly interested in brain systems involved in sensory decision-making.
Want to learn more about how these researchers study mindfulness? You can find Dale’s paper here!
Keeping your brain's symphony in sync
or technically,
Weakly correlated local cortical state switches under anesthesia lead to strongly correlated global states
[See original abstract on Pubmed]
Dr. Brenna Shortal was one of the two lead authors of this publication. Her graduate and undergraduate research focused on understanding the neurological mechanisms of consciousness, and she has published a number of papers on the topic. While she was a student at UPenn, Dr. Shortal was the director of Brains in Briefs, and her passion for science communication led her to pursue a career as a medical writer for Red Nucleus following her graduation in 2021. She hopes to continue working to communicate and advocate for scientific research to broad audiences.
or technically,
Weakly correlated local cortical state switches under anesthesia lead to strongly correlated global states
[See Original Abstract on Pubmed]
Authors of the study: Ethan B Blackwood, Brenna P Shortal, Alex Proekt
The most complicated piece of machinery you will ever encounter is sitting right between your ears: your brain. Our ability to move, sense, and think is thanks to billions of individual neurons that interact in varied and complicated ways. With this level of complexity, it’s miraculous that our brains work at all, let alone as well or as long as they do. Even more impressively, when our brain gets knocked off track, like from a seizure or anesthesia, it can quickly go back to typical patterns of activity. How does such a complex thing keep itself in sync?
Ethan Blackwood is a fifth-year neuroscience graduate student in the lab of Dr. Alex Proekt. Before coming to Penn and as a rotation student with Dr. Proekt, his research focused on how neural oscillations ("brain waves") change over time or with stimulation and what this means for behavior. More recently, he has been zooming in to the individual neuron level and studying how the firing of large groups of neurons changes during learning.
Neuroscience PhD student Ethan Blackwood and Drs. Brenna Shortal and Alex Proekt at the University of Pennsylvania sought to answer this question by studying brain activity in rats under anesthesia. Anesthesia is a useful way to study the coordination of brain activity because it is easy to put animals under anesthesia in the lab and because researchers already know a lot about the patterns of brain activity that occur when people are under anesthesia. The team studied this phenomenon in rats because they were able to directly record the activity of the neurons in the rat’s brain, something that is rarely possible in the human brain.
The team had two ideas about how the brain keeps itself in sync. Their ideas are easiest to understand if we think of the brain as a symphony with your neurons as the musicians. Just like an orchestral piece comes together because the musicians move in sync from one part of the music to the next, so too do the groups of neurons in your brain. The researchers’ first idea about how the brain might keep its symphony together was that there is a conductor who dictates how all the groups of neurons behave. The second possibility was that there is no conductor, but nearby neurons listen to each other so that the whole orchestra stays together.
To distinguish between these two possibilities, the team recorded a kind of brain signal called a local field potential in two parts of the rat brain. They did this by placing electrodes in the rat’s brain and listening to the activity of nearby neurons. This is like listening to a few microphones placed in the cello and violin sections to understand how the whole orchestra works. Each microphone captures sound produced by several nearby musicians, but it can’t capture the whole orchestra’s sound.
The team started by identifying what musical melodies, which they call brain states, each electrode recorded and noting when the nearby neurons switched from one state to the next. By doing this for all the electrodes, they showed that there were only a small number of brain states that the neurons played, and the same states appeared in different rats. The relatively small number of brain states they found is something other neuroscientists have observed, and it’s key to how the brain keeps itself in sync. If every musician in the orchestra played their own tune, it would be hard to make sense of what was going on. However, by moving through different sections of the same piece of music in sync, the instruments create a beautiful piece of music together. The same is true of your brain’s symphony. Rather than coordinating billions of songs, each sung by different neurons, your brain’s symphony sings just a few, transitioning between a small number of brain states over time.
Now that they had their brain recordings, the team could see which of their two proposals about how the neural symphony stays in sync was true. If their first prediction, that there is a conductor that signals when to transition from one state to another, was true, the team expected to see all the groups of neurons transitioning between states at similar times. On the other hand, if their second prediction was true, that the neural symphony stays in sync by listening to nearby neurons, the researchers would expect to see groups of nearby neurons transitioning between themes mostly together, with nearby neurons more likely to move together than neurons that are further apart. When they measured the neurons’ activity, they found that transitions between states measured on different electrodes corresponded only weakly to each other, but that the closer the electrodes were, the more the state transitions were related. This supported their second prediction, that neurons listen to their neighbors to decide when to transition from one state to another.
This is an exciting step toward understanding how the brain coordinates the movements between states that help keep our complex brains in sync. Understanding this process is important because it can help us develop therapies that mimic it for patients whose brain activity can’t always keep up healthy patterns, such as seizure patients. Beyond medical uses, understanding nature’s elegant solution to managing the complexity of brain signaling can teach us how to build computer systems and models that can handle increasingly more complexity to do things like power robots. And if none of these applications excite you, hopefully you can appreciate the wonder of understanding a little more about what makes us tick and how our neural symphonies stay in sync.
About the brief writer: Catrina Hacker
Catrina Hacker is a PhD candidate working in Dr. Nicole Rust’s lab. She is broadly interested in the neural correlates of cognitive processes and is currently studying how we remember what we see. She also co-directs PennNeuroKnow.